Minimally invasive left ventricular assist device placement
Introduction
Heart failure remains to be a major global problem with over 26 million people suffering from heart failure around the world including approximate 6 million in United States and 13 million in China alone (1,2). With the advancement in mechanical circulatory support and the continued limitation of donor supply for heart transplantation, the use of left ventricular assist device (LVAD) as bridge-to-transplant and destination therapy has increased significantly in recent years (3). It is hence important to be familiar with the surgical techniques of LVAD placement, but more importantly to further advance the techniques in order to optimize patient outcomes. Multiple studies have shown minimally invasive cardiac surgeries, including cardiac valves operations can improve outcomes with shorter hospital stay, decreased post-operative bleeding, reduced blood transfusion requirement, faster ambulation/recovery rate and lower total hospital cost (4-7). The technology of mechanical circulatory support has significantly improved in the last decade. Data have shown, the current generation of LVADs are more durable, and are associated with fewer complications and better survival (8). The current generations of centrifugal continuous-flow LVADs are smaller and have allowed minimally invasive surgical approaches. In this review, we describe our surgical technique of minimally invasive LVAD placement (HeartWare HVAD, Framingham, MA, USA).
Technique
Preoperative evaluation and patient selection
In addition to the standard pre-LVAD implantation evaluation, echocardiography, chest radiography and noncontrast chest computed tomography are further examined. Echocardiography helps identify patients with valvular abnormality who need concomitant valve procedure and would preferably be approached through a standard midline sternotomy. In the presence of left ventricle thrombus, an on-pump approach should be performed for thrombus removal. Patent foramen ovale (PFO) does not exclude minimally invasive approach. We generally do not close PFO for continuous-flow LVAD placement, unless it becomes hemodynamically significant after LVAD implantation, then percutaneous PFO closure utilizing the Amplatzer occluding device can be performed. Patients with right heart failure may actually benefit from a non-sternotomy approach, in which the intact pericardium over the right ventricle can protect the right ventricle from acute unrestricted dilation after LVAD placement. The chest radiography and noncontrast chest computed tomography are helpful in determining which intercostal space to be used and whether an antero-lateral (smaller left ventricle) vs. lateral (rotated and displaced left ventricle) mini-thoracotomy should be utilized. Most of the time, the 5th or 6th intercostal space is used for the access of the left ventricular (LV) apex. Chest computed tomography will also be useful for the evaluation of the outflow aortic anastomosis site and to rule out aortic calcification and aneurysm. Other outflow anastomosis sites can be utilized if the ascending aorta is prohibitive for the outflow graft anastomosis. At our center, we use the left subclavian artery and descending aorta as alternative sites for outflow graft anastomosis. But their approaches and approaches for patients with previous sternotomies will not be discussed in this article.
Surgical technique
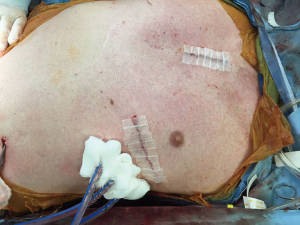
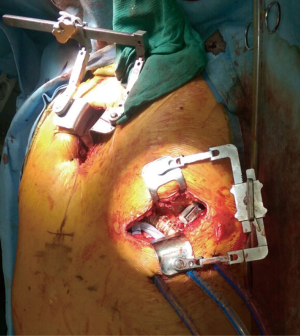
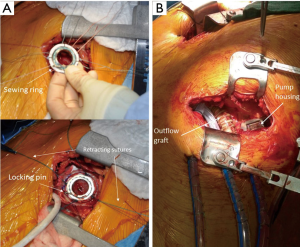
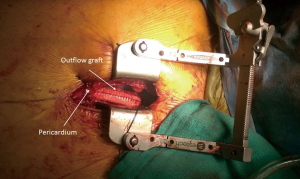
Intra-operative transthoracic echocardiography is used to identify the LV apex location and to determine the optimal location and intercostal space for the left thoracotomy. For a dilated large left ventricle, the thoracotomy site needs to be more lateral, whereas for a small left ventricle, the thoracotomy incision tends to be more anterior and medial. A 6 cm thoracotomy incision is usually performed in the fifth or sixth intercostal space (Figure 1). Upon entering the chest, the pericardium is identified, divided, and a pericardial cradle is created with pericardial sutures to expose the LV apex. The LV apex sewing ring is sewn on to the apex off-pump with 2-0 ethibond (Ethicon, Somerville, NJ, USA) pledgeted sutures in a mattress fashion (Figure 2A). The locking pin of the sewing ring should be oriented anteriorly to allow easy access after the inflow cannula is inserted. A 4 cm upper hemi-sternotomy is performed to expose the ascending aorta (Figure 1). If cardiopulmonary bypass is planned, we use the distal ascending aorta for arterial cannulation to assure adequate cerebral perfusion. Femoral vein is used for a percutaneous venous cannulation utilizing the Seldinger technique with transesophageal echocardiography guidance. Off-pump technique can also be used in selected patients. Furthermore, instead of an upper hemi-sternotomy, a right second intercostal anterior thoracotomy can be performed if the ascending aorta demonstrated a right-sided curvature on preoperative chest computed tomography. This approach is especially beneficial in bridge-to-transplant patient in whom the sternal incision is spared for future heart transplantation. If cardiopulmonary bypass is planned, it is initiated before the coring of the LV apex, after the patient is placed in Trendelenburg position and after the sewing ring is secured. After coring, the LV should be thoroughly examined for LV thrombus and thrombus removal should be performed. For off-pump approach, sometimes we give 30 mg bolus of adenosine to induce a brief period of bradycardia, to decrease LV ejection and to minimize bleeding during the placement of the inflow cannula. Lidocaine and magnesium are given ahead of time to decrease LV arrhythmogenicity during the off-pump approach. The outflow graft is tunneled within the pericardium (Figure 2B) and out of the upper hemi-sternotomy or right second intercostal anterior thoracotomy incision. De-airing maneuver is performed through the outflow graft. It should be noted that half of the strain relief on the outflow graft is removed before the insertion of the inflow cannula to allow easier outflow graft placement and better angulation of the outflow graft within the chest curving toward the right chest away from the sternum. A side-biter clamp is placed on the proximal ascending aorta for the end-to-side outflow graft anastomosis at the right lateral aspect of the proximal ascending aorta using a 4-0 polypropylene suture (Figure 3). The outflow graft is positioned curving around from the right chest away from the sternum. The outflow graft anastomosis site is covered with pericardium and pericardial fat. The driveline can be tunneled through the right or left upper quadrant of the upper abdomen. Cardiopulmonary bypass is then weaned (if on-pump) in the standard fashion and the LVAD pump is started. A piece of Gore-Tex pericardial membrane is used to cover the strain relief for future re-entry, e.g., heart transplantation. The thoracotomy and hemi-sternotomy are closed in the standard fashion (Figures 4,5).
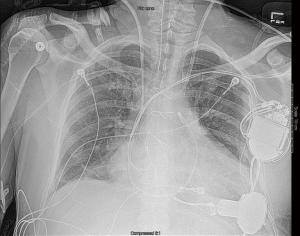
Discussion
The use of LVAD has increased significantly along with the rapidly expanding heart failure patient population. Multiple studies have shown minimal invasive cardiac surgery improves outcomes with reduced surgical trauma and complication rates, e.g., less post-operative bleeding, reduced blood transfusion requirement, faster recovery rate and decreased hospital cost. For bridge-to-transplant patient, avoiding a full sternotomy can make subsequent LVAD explantation and heart transplantation less technically challenging. The use of off-pump LVAD implantation approach allows the avoidance of cardiopulmonary bypass and will decrease the incidence of vasoplegia and coagulopathy post-operatively. With the advancement of the current generation LVADs, they have been shown to be more durable with good outcomes comparable to heart transplantation (3,9,10). The smaller size of the current generation LVADs have made less invasive LVAD placement feasible. The future generations of LVADs are going to be even smaller (e.g., Heartware MVAD), and the development of new minimally invasive surgical techniques and instrumentations for the future generations of LVADs are already in progress at various laboratories, including the one at our institution. Further large prospective, randomized studies, including the current ongoing HeartWare LATERAL trial, are needed to further demonstrate the potential advantages and disadvantages of minimally invasive LVAD placement. This less invasive approach is technically more challenging than is full sternotomy. Therefore, such approach should be performed by experienced VAD surgeons at high volume centers to ensure the highest quality of LVAD surgery.
Conclusion
With the current generation of continuous-flow left ventricular assist device, minimally invasive LVAD placement, along with off-pump implantation, is feasible and reproducible and may improve patient outcomes. It is crucial for all heart failure surgeons to be familiar with the standard surgical technique of LVAD placement, but also to further advance the surgical technique of implantation to optimize patients outcomes. The upcoming advancement of LVAD technology and miniaturization of mechanical circulatory support will only further advance the success of LVAD therapy and will benefit more and more patients especially with the rapidly growing heart failure population worldwide.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethical committee. Written informed consent was obtained from the patient for publication. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:399-410. [PubMed]
- Gu K, Chang Y, Gao B, et al. Development of ventricular assist devices in China: present status, opportunities and challenges. Eur J Cardiothorac Surg 2014;46:179-85. [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015. [Epub ahead of print]. [PubMed]
- McClure RS, Athanasopoulos LV, McGurk S, et al. One thousand minimally invasive mitral valve operations: early outcomes, late outcomes, and echocardiographic follow-up. J Thorac Cardiovasc Surg 2013;145:1199-206. [PubMed]
- Iribarne A, Easterwood R, Russo MJ, et al. Comparative effectiveness of minimally invasive versus traditional sternotomy mitral valve surgery in elderly patients. J Thorac Cardiovasc Surg 2012;143:S86-90. [PubMed]
- Iribarne A, Easterwood R, Russo MJ, et al. A minimally invasive approach is more cost-effective than a traditional sternotomy approach for mitral valve surgery. J Thorac Cardiovasc Surg 2011;142:1507-14. [PubMed]
- Galloway AC, Schwartz CF, Ribakove GH, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg 2009;88:1180-4. [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [PubMed]
- Schumer EM, Ising MS, Trivedi JR, et al. Early Outcomes With Marginal Donor Hearts Compared With Left Ventricular Assist Device Support in Patients With Advanced Heart Failure. Ann Thorac Surg 2015;100:522-7. [PubMed]
- Trivedi JR, Cheng A, Singh R, et al. Survival on the heart transplant waiting list: impact of continuous flow left ventricular assist device as bridge to transplant. Ann Thorac Surg 2014;98:830-4. [PubMed]
Cite this article as: Cheng A. Minimally invasive left ventricular assist device placement. J Vis Surg 2015;1:25.