Robotic upper lobe segmentectomy: technical pearls
Introduction
While the incidence of lung cancer has been steady over the last several years, it remains the leading cause of cancer related mortality in the United States for both men and women (1). Fortunately, there has been a significant decrease in mortality from non-small cell lung cancer (NSCLC) over the past two decades, driven by developments in targeted therapies and immunotherapy (2), and a trend towards increase in early-stage diagnosis with the adoption of low-dose computed tomography (CT) screening for high-risk individuals (3). The increase in the incidence of early-stage disease has implications for surgeons, who will be presented with more cases of up-front resectable disease.
A landmark trial by Ginsberg and colleagues in 1995 established lobectomy as the standard of care for early-stage NSCLC (4). Early experience with open segmentectomy had been reported as many as 20 years earlier (5), with the goals of achieving the same oncologic results as lobectomy while preserving respiratory function. However, concerns over increased local recurrence and lower survival compared to lobectomy delayed broader adoption of the technique (6,7). More recent retrospective studies, though, have shown no significant differences in local recurrence or survival between segmentectomy and lobectomy (8-11). Importantly, there are two ongoing clinical trials, CALGB 140503 (12) and JCOG0802/WJOG4607L (13) comparing lobectomy to segmentectomy. The CALGB/Alliance trial 140503 randomized 697 patients to lobectomy or sublobar resection (segmentectomy or wedge) and noted no difference in peri-operative morbidity or mortality between arms (12). The Japanese trial, JCOG0802/WJOG4607, was larger with 1,106 patients randomized between lobectomy and segmentectomy (no wedge resections) and similarly reported equivalent short-term outcomes between procedures (13). The primary outcome, 5-year overall survival, was reported at the American Association for Thoracic Surgery conference in 2021 and was significantly better in the segmentectomy arm [hazard ratio (HR) 0.663]. Segmentectomy trended toward improved survival in all subgroups evaluated, but significant differences were not seen with respect to post-operative pulmonary function. Local recurrences were more common in the segmentectomy group, but most were salvageable and did not lead to increased mortality (14).
Traditionally, anatomic lung resection has been approached via thoracotomy. Video-assisted thoracic surgery (VATS) and robotic thoracic surgery have been introduced as minimally invasive alternatives to open chest surgery. When compared to open, both minimally invasive approaches have several advantages, including reduced pain, lower complication rates, and shorter hospital stays (15-20). Between the two minimally invasive approaches, the proposed advantages of the robot are the 3-dimensional visualization, greater degrees of freedom with smaller instruments, filtration of tremor, and improved ergonomics (21). The disadvantages compared to VATS are lack of tactile feedback and increased cost (22). However, as surgeon experience with the robotic technique improves, especially with minimizing instrument use, decreasing operative time and shortening hospital length of stay, the total cost becomes similar. While some centers have reduced cost with the robotic approach (23), others have demonstrated higher costs associated with robotic lobectomy compared to VATS, as in the recent RVLob trial (24).
Recent retrospective studies have evaluated the clinical outcomes between robotic and VATS segmentectomy. In a study by Zhang and colleagues (25), 257 propensity matched patients underwent robotic segmentectomy and VATS segmentectomy. There was no difference in conversion rate, operative time, blood loss, complication rates, or length of stay. While the robotic approach was more expensive, there was a trend towards increased nodal sampling in the robotic group. Similarly, a recent meta-analysis of 14 studies comparing robotic to VATS lobectomy and segmentectomy found a lower 30-day mortality rate and fewer conversions to thoracotomy in the robotic group. There was also no difference in complication rates, length of stay, or nodal sampling (26). Furthermore, the finer control of the robot is an advantage when performing difficult dissections. For example, Zhou and colleagues reported an increase in segmentectomy at their center over time, and particularly an increased use of the robot for complex segmentectomies. Despite the complex cases, the robotic approach was associated with low perioperative morbidity and mortality, and there were no conversions to open (27).
Preoperative evaluation and considerations
A thorough pre-operative evaluation is necessary for any patient being considered for lung resection. Evaluation begins with the standard preoperative studies for patients with suspected or biopsy-proven lung cancer. Positron emission tomography-CT, mediastinal staging in most patients with solid lesions, and pulmonary function testing are required. Other patients may need additional testing, such as preoperative cardiac risk evaluation, or brain magnetic resonance imaging for suspected metastatic disease. Care should also be coordinated with a medical oncologist. Once a tumor is deemed amenable to surgery, an anatomic resection is then chosen. In general, segmentectomy can be a reasonable choice for small, peripheral tumors that are <2 cm in diameter, margins >2 cm, and no evidence of nodal disease on staging (28,29). A key aspect of surgical planning and choosing between segmentectomy and a larger resection is a preoperative CT scan. We use a high-resolution, intravenous contrast-enhanced chest CT scan when evaluating a lesion for resection. Three-dimensional CT reconstruction is also useful when available. We specifically identify the segment containing the lesion, and measure the anticipated margins (2 cm). If the lesion is less than 2 cm from the border of the segment, we then discuss taking the neighboring segment versus the lobe if it would be tolerated. In addition to assessing the margins, we review the vascular and bronchial anatomy of the segment planned for resection, specifically looking for variant anatomy. Reviewing the preoperative CT scan for these details is imperative for surgical planning.
Segmentectomy is also very useful for suspicious ground glass opacities and subsolid lesions, especially as these are often not in the periphery of the lung. It may also be beneficial in patients with advanced age, who are more frail and have reduced cardiopulmonary reserve. We perform robotic segmentectomy on most patients who meet these criteria. However, in patients with small, solid lesions who can tolerate a lobectomy, we send the station 11 node for frozen section at the beginning of the case and proceed to a lobectomy if positive (30). We do not routinely do this for subsolid or ground glass opacity lesions.
Surgical technique for robotic segmentectomy
The patient is first positioned supine on the operating room table and undergoes general anesthesia and endotracheal intubation with a double lumen tube for lung isolation. A flexible bronchoscopy is mandatory to evaluate for endobronchial disease not appreciated on preoperative imaging, and to define the segmental anatomy. It also confirms appropriate positioning of the double lumen tube.
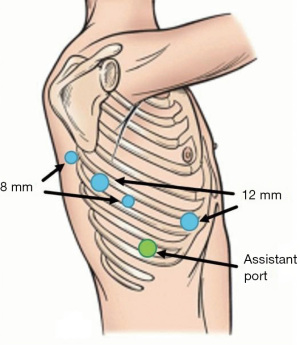
After securing the endotracheal tube, the patient is repositioned in lateral decubitus position and the bed is flexed. Both arms are placed out in front of the patient, flexed at the shoulder to 90 degrees and resting on padded arm boards. The upper arm should be on an elevated arm board. The patient is then prepped and draped, and sites are chosen for the robotic ports (Figure 1). We first place the 8-mm camera port in eighth intercostal space via a 1-cm incision in the posterior axillary line for upper lobe operations. A 12-mm stapler port is placed 8 cm posterior to the camera port, in the same intercostal space. A second 8-mm port is placed further posteriorly, 4 cm from the spine, over the level of the major fissure to optimize lung retraction. A second 12-mm stapler port is placed as medial as possible to the camera port, at the cardiophrenic recess near the sternum. For most upper lobe segments, it is our preference to use two 12-mm staple ports, as it allows for easier access to the vessels and bronchus having two angles to fire the stapler. However, it is also acceptable to use a single 12-mm port and three 8-mm ports for any upper lobe segmentectomy. For posterior segments, only the anterior staple port is required, so we use an 8-mm port in place of the more posterior 12-mm port. An assistant port is placed inferiorly in the tenth intercostal space, triangulating between the two most anterior ports. The camera port is placed first, and the camera is then inserted into the thoracic cavity for placement of the remaining ports under direct vision. We typically use the 30-degree camera.
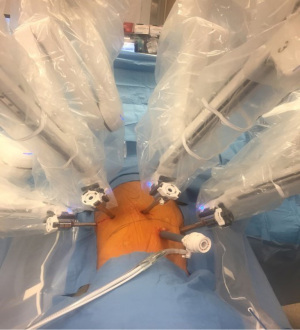
Once the ports have been placed, the robot can be docked (Figure 2). The robot is brought in at a perpendicular angle to the patient. After docking, we use CO2 insufflation and insufflate to 8 mmHg. Once the robot is docked and the camera and instruments are appropriately positioned towards the target anatomy, the surgeon moves to the console to begin the dissection.
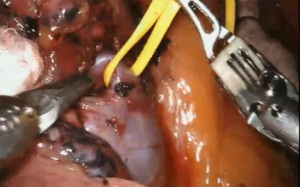
We first inspect the pleura for metastases. We then begin with a posterior mediastinal dissection. The inferior pulmonary ligament is divided. Next, as we continue to the posterior dissection, we remove stations 8 and 9, then clear out the station 7 nodes. The lung is then retracted caudad to allow for dissection of stations 2R and 4R (right sided resections) or stations 5 and 6 (left sided resections). For upper lobe segments, we then perform a suprahilar dissection to clear off the pulmonary artery (PA). At this point, we also sample the station 10 nodes and send them for frozen section. If they are positive for malignancy, a lobectomy is performed. At this point, we proceed based on the segmentectomy to be performed. In general, the hilar structures are identified and divided, taking care to preserve the segments to be left behind. After division of the hilar structures, the parenchyma is divided with serial stapler firings. The boundaries for the parenchymal division have traditionally been determined by gentle insufflation of the lung after bronchial division, leaving the affected segment uninflated. Intraoperative bronchoscopy with selective jet ventilation is also an option to identify the intersegmental planes. Our preference is to utilize the near-infrared technology offered on the DaVinci robot (FirelyTM) and infuse 5 mg indocyanine green (ICG) intravenously to identify the borders between the ischemic segment (after PA division), and the perfused remaining segments (Figure 3).

Right upper lobe (RUL) apical segmentectomy (RS1)
After the mediastinal lymph node dissection is complete, we retract the lung posteriorly to expose the anterior hilum. The pleura over the hilar structures is opened. The upper lobe vein is identified and dissected out until the division of the apical vein is encountered and divided (Figure 4). This creates the view to the apical branch of the PA, which is divided next. Before dividing the segmental artery, ensure there is no recurrent A2 branch, which should be preserved. After division of the artery, there is improved access to the segmental bronchus, which is also encircled and divided. If there is difficulty exposing the bronchus, the lung can be retracted anteriorly to expose the posterior aspect of the bronchus. After division of the bronchus, the remaining parenchyma is then divided with the robotic stapler.
RUL posterior segmentectomy (RS2)
We begin by retracting the lung anteriorly, beginning with a posterior view of the hilum. We first identify the triangle between the bronchus intermedius and RUL bronchus. We then remove the station 11 node, or “sump node”, which usually exposes the posterior segmental artery to the RUL. If there is poor exposure of the vessels and bronchus at this point, the posterior oblique fissure may need to be divided before proceeding. The posterior segmental artery is then dissected free and stapled, but again, watch for a recurrent A2 branch, which should be preserved if present. The same is repeated for the posterior segmental vein. Next, the bronchus is dissected and the posterior segment and apical and anterior segments are differentiated, although it may be necessary to clear off any nodes around the bronchus to better visualize the segmental anatomy. If needed, a bronchoscope can be inserted at the time of bronchial dissection and the FireflyTM near-infrared imaging can be utilized to visualize the bronchoscope in the appropriate bronchial segment. After confirming the anatomy, retract the bronchus stump cephalad to expose the PA to the middle and lower lobe to ensure their preservation as the remaining parenchyma is stapled to complete the segmentectomy.
RUL anterior segmentectomy (RS3)
After the mediastinal nodal harvest, we begin with an anterior dissection in the horizontal fissure. We identify the superior pulmonary vein in the hilum, then carry dissection distally until the division between the central vein and the apical segmental vein is encountered. The main PA and middle lobe artery are then identified to prevent damage during the dissection of the venous branches, which lie just anterior to them. We then return to the dissection of the venous trunk. The central vein is exposed distally until the anterior segmental branch is encountered. It is then encircled and divided. The horizontal fissure needs be completed with stapler loads to expose B3, and sometimes the anterior segmental artery (A3). A3 is then identified as the most inferior branch off of the common trunk to the apical and anterior segments. It is encircled and divided. Note that in some cases, it may arise directly off of the main PA or the truncus intermedius. After division of the artery, the bronchus is divided. The remaining parenchyma is then taken with serial firings of the stapler.
Left upper lobe (LUL) tri-segmentectomy (LS1+2+3)
After harvesting the mediastinal nodes, the anterior pleura over the left main PA is opened. The plane between the anterior surface of the artery and the superior pulmonary vein is dissected out, which allows access to the trunk draining the trisegment, usually just proximal to the lingular vein. Once the lingular vein is identified and preserved, the vein to the upper lobe trisegment is encircled and divided. The artery to the trisegment is then dissected free and divided, taking care to identify a separate posterior branch (A1+2 c or A1+2 b+c), which occurs frequently. After the division of the artery, the segmental bronchus is divided. In each step, care is taken to preserve the nearby branches to the lingular segment. These branches are commonly pre-bronchial and can be a potential source of bleeding if not preserved during the dissection. Once the hilar structures have been safely divided, the parenchyma is then divided with serial firings of the stapler.
Lingulectomy (LS4–5)
We start by identifying the pulmonary vein to the lingula, from an anterior approach. After confirming the anatomy to ensure preservation of the other segments, the lingular venous drainage is divided. We then identify the branches of the PA to the lingula and similarly divide them. In a minority of cases, there is an A4+5 branch arising from the posterior trunk or main PA. Division of the arterial branches exposes the lingular bronchus well, and it is dissected and divided. The remaining parenchyma is stapled to complete the lingulectomy.
LUL Apicoposterior (S1+S2) segmentectomy
After harvesting the mediastinal nodes, the lung is retracted posteriorly to expose the anterior mediastinal pleura. The pleura over the left main PA is opened. The artery is carefully followed laterally until the apicoposterior branch of the pulmonary vein is encountered as it crosses the artery anteriorly. The vein is then dissected proximally until it drains into the superior pulmonary vein. Then, the branches from the anterior and lingular venous segments can be identified and preserved. The apicoposterior segmental vein is then dissected circumferentially and divided. This improves exposure to the left main PA, which is dissected distally until the trunk to the apicoposterior segments is encountered. Dissection is continued to identify the branches to the anterior and lingular segments, which will be preserved. The segmental artery is then encircled and divided. This will expose the apicoposterior bronchus, which is similarly dissected and divided. The bronchus may be clamped before firing the stapler, and the lung may be insufflated to confirm only the apical and posterior segments are taken. After the bronchus is divided, the remaining parenchyma is taken with a stapler.
LUL anterior segmentectomy (S3)
After the mediastinal nodal harvest and posterior dissection, we expose the superior pulmonary vein, following it distally until the division between the apicoposterior venous branch and anterior venous branch. The V3 branch is then encircled and divided. This usually exposes the anterior artery branch A3, which is deep and superior to the V3 branch that was divided. For better exposure, the V2 can be retracted with a vessel loop such that the A3 branch can be easily dissected and divided. The B3 bronchus remains and is similarly dissected out and divided. The horizontal fissure may be completed with stapler loads if necessary to improve exposure to the arterial and bronchial structures. After division of the bronchus, the remaining parenchyma is then taken with serial firings of the stapler.
It is important to note that, in the rare event of major vascular injury or other emergency, the robot should be undocked after pressure is applied to the vascular injury with a sponge and the bleeding is controlled. Conversion to thoracotomy can be performed as needed to address the injury.
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and the accompanying video (Video 1).
Postoperative care
Enhanced recovery after surgery (ERAS) protocols have been described for thoracic surgery (31,32). The key elements involve minimizing intravenous fluids postoperatively, early feeding, early mobilization, and avoiding opiate analgesia when possible. This has been shown to reduce length of stay and morbidity (31), with no increased risk for readmission (33). We implement a standard ERAS protocol for our robotic segmentectomy patients. Scheduled acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) are given for pain, unless there is a contraindication. We try to limit narcotic use, but oxycodone can be used for breakthrough pain. Patients are given a regular diet postoperatively, and early ambulation is encouraged. Early chest tube removal and discharge is encouraged with a goal of discharge on postoperative day 1 or 2.
Summary
The robotic approach to upper lobe segmentectomy offers excellent visualization of the lung anatomy and allows for precise dissection. It is a safe and oncologically-effective alternative to lobectomy for appropriate patients. Usual preoperative workup including tumor staging and pulmonary function testing is mandatory. Understanding of classic and variant segmental anatomy is important for safe dissection, and preservation of the remaining lobe. Postoperatively, an ERAS protocol can be implemented to optimize recovery and promote early discharge.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hiran Fernando and John Lazar) for the series “Innovations in Robotic VATS and Bronchoscopic Procedures” published in Journal of Visualized Surgery. The article has undergone external peer review.
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-60/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-60/coif). The series “Innovations in Robotic VATS and Bronchoscopic Procedures” was commissioned by the editorial office without any funding or sponsorship. HA receives consulting fees when acting as a proctor for training robotic surgeons, via Intuitive Surgical; they have also sponsored the attendance at a national teaching conference on emerging technology in robotic surgery. LS is a speaker and serves on the advisory board for Intuitive Surgical. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and the accompanying video.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020;383:640-9. [Crossref] [PubMed]
- Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920-31. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Jensik RJ, Faber LP, Milloy FJ, et al. Segmental resection for lung cancer. A fifteen-year experience. J Thorac Cardiovasc Surg 1973;66:563-72. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [Crossref] [PubMed]
- Razi SS, Nguyen D, Villamizar N. Lobectomy does not confer survival advantage over segmentectomy for non-small cell lung cancer with unsuspected nodal disease. J Thorac Cardiovasc Surg 2020;159:2469-2483.e4. [Crossref] [PubMed]
- Subramanian M, McMurry T, Meyers BF, et al. Long-Term Results for Clinical Stage IA Lung Cancer: Comparing Lobectomy and Sublobar Resection. Ann Thorac Surg 2018;106:375-81. [Crossref] [PubMed]
- Zhang L, Gao S. Robot-assisted thoracic surgery versus open thoracic surgery for lung cancer: a system review and meta-analysis. Int J Clin Exp Med 2015;8:17804-10. [PubMed]
- Yang CF, Kumar A, Gulack BC, et al. Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis. J Thorac Cardiovasc Surg 2016;151:1380-8. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Asamura H, Okada M, Saji H, et al. Randomized trial of segmentectomy compared to lobectomy in small-sized, peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). American Association for Thoracic Surgery Annual Meeting. Virtual, 2021.
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Hanna WC, de Valence M, Atenafu EG, et al. Is video-assisted lobectomy for non-small-cell lung cancer oncologically equivalent to open lobectomy? Eur J Cardiothorac Surg 2013;43:1121-5. [Crossref] [PubMed]
- Zhang Z, Zhang Y, Feng H, et al. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2013;44:407-14. [Crossref] [PubMed]
- Velez-Cubian FO, Ng EP, Fontaine JP, et al. Robotic-Assisted Videothoracoscopic Surgery of the Lung. Cancer Control 2015;22:314-25. [Crossref] [PubMed]
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [Crossref] [PubMed]
- Nawalaniec JT, Elson M, Reznik SI, et al. Training Cardiothoracic Residents in Robotic Lobectomy Is Cost-Effective With No Change in Clinical Outcomes. Innovations (Phila) 2022;17:127-35. [Crossref] [PubMed]
- Veronesi G. Robotic surgery for the treatment of early-stage lung cancer. Curr Opin Oncol 2013;25:107-14. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 1095-6. [Crossref] [PubMed]
- Coyan GN, Lu M, Ruppert KM, et al. Activity-Based Cost Analysis of Robotic Anatomic Lung Resection During Program Implementation. Ann Thorac Surg 2022;113:244-9. [Crossref] [PubMed]
- Jin R, Zheng Y, Yuan Y, et al. Robotic-assisted Versus Video-assisted Thoracoscopic Lobectomy: Short-term Results of a Randomized Clinical Trial (RVlob Trial). Ann Surg 2022;275:295-302. [Crossref] [PubMed]
- Zhang Y, Chen C, Hu J, et al. Early outcomes of robotic versus thoracoscopic segmentectomy for early-stage lung cancer: A multi-institutional propensity score-matched analysis. J Thorac Cardiovasc Surg 2020;160:1363-72. [Crossref] [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]
- Zhou N, Corsini EM, Antonoff MB, et al. Robotic Surgery and Anatomic Segmentectomy: An Analysis of Trends, Patient Selection, and Outcomes. Ann Thorac Surg 2022;113:975-83. [Crossref] [PubMed]
- Nomori H, Mori T, Ikeda K, et al. Segmentectomy for selected cT1N0M0 non-small cell lung cancer: a prospective study at a single institute. J Thorac Cardiovasc Surg 2012;144:87-93. [Crossref] [PubMed]
- Villamizar N, Swanson SJ. Lobectomy vs. segmentectomy for NSCLC (T<2 cm). Ann Cardiothorac Surg 2014;3:160-6. [PubMed]
- Raman V, Jawitz OK, Voigt SL, et al. The Effect of Tumor Size and Histologic Findings on Outcomes After Segmentectomy vs Lobectomy for Clinically Node-Negative Non-Small Cell Lung Cancer. Chest 2021;159:390-400. [Crossref] [PubMed]
- Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843-52. [Crossref] [PubMed]
- Martin LW, Sarosiek BM, Harrison MA, et al. Implementing a Thoracic Enhanced Recovery Program: Lessons Learned in the First Year. Ann Thorac Surg 2018;105:1597-604. [Crossref] [PubMed]
- Patel DC, Leipzig M, Jeffrey Yang CF, et al. Early Discharge After Lobectomy for Lung Cancer Does Not Equate to Early Readmission. Ann Thorac Surg 2022;113:1634-40. [Crossref] [PubMed]
Cite this article as: Nawalaniec J, Auchincloss H, Schumacher L. Robotic upper lobe segmentectomy: technical pearls. J Vis Surg 2023;9:7.