Four years’ experience in uniportal video-assisted thoracoscopic surgery for major lung resections: influence of silicosis in clinical outcomes
IntroductionOther Section
Currently video-assisted thoracoscopic surgery (VATS) is established as the first choice surgical thoracic approach for diagnosing and treatment of lung diseases. It is for this reason that any thoracic intervention that could benefit from the advantages, ought to use this approach (1-3). The latest step in the evolution of the technique has been the use of a single 4 to 5 cm incision for major anatomic lung resections. This access was initially described by Rocco for diagnostic and therapeutic purposes and later successfully developed by Gonzalez-Rivas for lobectomies (4-7) showing good perioperative results when performed by surgeons experienced in double-port technique and anterior thoracotomy.
It has been suggested that the learning curve usually demands approximately 50 VATS lobectomies. Petersen et al. in 2012 suggested general recommendations for the introduction of a VATS lobectomy program. The recommendations include: experience in performing open lobectomy by anterior thoracotomy, experience in completing more than 100 minor VATS procedures, attendance at formal courses in VATS lobectomy, choosing a specific VATS modality (anterior or posterior, using 2–4 ports), selection of the patients, introduction of the element of resection step-by-step, and making a prospective data collection (8).
Even though one of the main recommendations is the selection of cases during the learning curve, the high incidence of silicosis in our region and our extensive experience in the surgical treatment of this type of patient by open approach, allowed us to include complex cases with complex pulmonary silicosis with lymph node involvement.
Crystalline silica (CS) is one of the most common minerals and a common particulate air pollutant in both working and living environments. Recent reports have indicated that more than 1.7 million workers in the United States, more than 2 million in Europe, and more than 23 million in China (9-11) have been occupationally exposed to CS dust. Lung cancer is considered one of the serious consequences of silica exposure.
Since acceptance of the feasibility of uniportal VATS for surgical treatment of lung cancer, progress and acceptance worldwide has not stopped; although there are still detractors regarding technical limitations of this approach (12), multiple complex surgeries as segmentectomy, bronchial sleeve and angioplasty were described (13,14), however up till now, the influence of silicosis in postoperative outcomes has not been documented. This is probably due to the low incidence of silicosis in general population. Only institutions, such as ours, with a high incidence of silicosis can draw on a sufficient number of cases to reach conclusions.
This paper gives an overview of the role of silicosis in results of perioperative and postoperative of lung resection surgery performed by the most up to date VATS approach: the uniportal VATS.
Materials and methodsOther Section
A 4-year retrospective descriptive study was performed between January 2012 and December 2015; 128 patients underwent uniportal VATS for major lung resection in the Department Of Thoracic Surgery Of Asturias University Central Hospital by a single surgeon. The institutional ethical review board of the Asturias University Central Hospital provided the approval for this study, and all patients provided written informed consent before the operation. The eligibility criteria for this study where: anatomical major lung resection and mediastinal lymph node sampling entirely performed using VATS by a single 4 to 5 cm incision without any rib spreader. The indications and selection criteria for a VATS lobectomy were based on the standard criteria for open thoracotomy including all histological types, and sizes except large tumors that are impossible to remove without rib spreading. Any patient with silicosis or other expected technical difficulty was excluded.
Preoperative evaluation
All patients underwent careful preoperative staging with a computed tomography scan within 8 weeks of surgery as well as pulmonary function tests. Additional diagnostic procedures such as brain-computed tomography scans, bone scans, or PET scans were considered according to the individual case, based upon patient symptoms and clinical findings. Mediastinoscopy was not routinely used in the preoperative evaluation of this group of patients. Flexible bronchoscopy was performed prior to surgical resection in all cases with centrally localized lesions. All patients diagnosed with lung cancer were staged according to the pathologic review, using the TNM classification of the American Joint Committee for Cancer Staging and the Revised International System for staging lung cancer, 7th edition (15).
Variables
The variables recorded in each patient were: demographic data such as age, sex and smoking habits, chronic obstructive pulmonary disease (COPD), pulmonary function test, presence of cardiopulmonary comorbidities, general characteristics of the tumor (size, position, stage and histological type), lymph node involvement, intraoperative findings, operating time, duration of chest tube, length of hospital stay, successfully completed or converted to thoracotomy, postoperative complications and 30-day mortality.
All complications were divided into two groups and analyzed. The group one (major complications) included the patients who underwent reoperation or some other surgical technique, or required intensive care unit life support. The second group (minor complications) includes the persistent air leak (>5 days), residual pleural effusion (present in post-discharge radiographic follow-up), pneumothorax (after chest tube removed), wound infection, and other complications not requiring extra medical care or surgical treatment. Complete follow-up data was obtained from regular radiological follow-up and personal records of the post-discharge visit.
Statistical analysis
All the data is reported as median or frequency, with associated interquartile ranges (IQR), 95% confidence intervals (CI), and P values calculated. Categorical data, both nominal and ordinal, were compared between groups using Fisher’s χ2 (exact) test. A probability value of less than 0.05 was considered to be statistically significant. SPSS, version 20.0 (IBM Corp) was used for the statistical analysis.
Operative technique and hospital course
All patients received general anesthesia using dual-lumen endotracheal tube, and were positioned in the lateral decubitus position with the bed flexed to increase the intercostal spacing. A 4 or 5 cm long incision was placed in the fifth intercostal space. A rib retractor was not used in any of the cases. In some overweight patients, a soft tissue retractor was used.
The surgeon and the assistant were positioned in front of the patient and had the same thoracoscopic vision during all the steps of the procedure.
For a panoramic view, a 10 mm/30° camera was used; which was introduced through the posterior part of the incision. However, the placement of the camera was modified at times according to the requirements of some particular step during the procedure.
In our first cases we used the longest and curved instruments for conventional open surgery mixed with thoracoscopy basic devices, but afterwards we added specific uniportal VATS instruments. Three or four instruments were used simultaneously for optimal exposure of the dissection.
After a VATS exploration, the hilar dissection was performed. The vessels and the bronchus were usually transected by staplers. In some particular cases, vascular clips where used (Hem-o-lok®, Teleflex, United States). For upper lobectomies, when possible, the arterial trunk was divided first and the fissureless technique was used if an uncompleted or absent fissure was found. We decided not to routinely make a section of the pulmonary ligament.
When lobectomy was completed, the lobe was removed avoiding direct contact with the wound by using a protective bag. A mediastinal lymph nodes sampling was performed routinely. The chest was drained with a straight chest tube (28 F on the first cases and 24 F on subsequent patients) through the thoracotomy incision. Epidural or paravertebral catheters were not used. The intercostal spaces were infiltrated with bupivacaine in all cases. Most patients were monitored in the intensive care unit overnight. Chest tube management and discharge planning were individualized based upon patient clinical characteristics and surgeon judgment. Chest tubes are usually removed when air leaks cease, the drainage decreases to less than 250 mL/d and the radiological control was normal. Patients were discharged after chest tube removal, followed by a radiological control. In cases of prolonged air leaks (>5 days), patients were discharged after placement of a Heimlich valve and ensuring stability of lung expansion.
ResultsOther Section
Patient and tumor characteristics
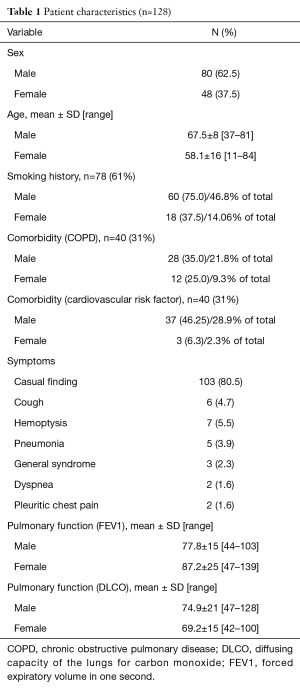
From January 2012 to December 2015, 128 patients were included in the single-port VATS anatomical lobectomy program by the same surgeon in the Department of Thoracic Surgery from Asturias University Central Hospital. Patient and tumor characteristics including age, sex, smoking habits, COPD, pulmonary function test results and the presence of cardiopulmonary comorbidities. Stage, comorbidity and pulmonary function are summarized in Table 1.
Full table
Of the 128 resections attempted [115 were completed (90%)], including left upper lobectomy in 38 patients (29%), right upper lobectomies in 34 patients (26%), left lower lobectomies in 26 patients (20%), right lower lobectomy in 17 patients (13%), right middle lobectomy in 5 patients (4%), left pneumonectomy in 2 patients (2%), upper and middle right bilobectomy in 2 patients (2%), lower and middle right bilobectomy in 2 patients (2%), right pneumonectomy in 1 patient (1%), and lingular segmentectomy in 1 patient (1%).
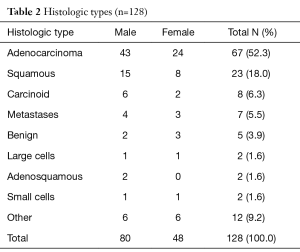
The most common histological types were primary lung adenocarcinoma in 67 patients (52%) and primary lung squamous cell carcinoma in 23 patients (18%). The remaining histologic distribution types are outlined in Table 2.
Full table
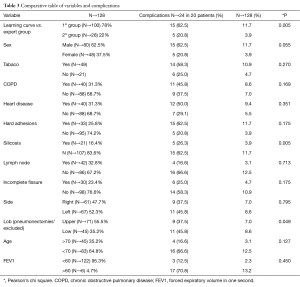
Twenty-one patients (16%) had complex silicosis, and 40 patients (31%) had diagnosis of COPD, which are indicative of postoperative complications. This association was also significant in patients with forced expiratory volume in one second (FEV1) <60%. A comparison between multiple variables and the complications is described in Table 3.
Full table
Intraoperative outcomes
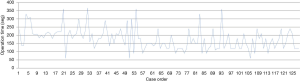
Of the 128 cases, there were 13 intraoperative complications that prevented completion of the resection through a uniportal approach. The conversions (lengthening the utility incision) from VATS to open thoracotomy were secondary to: uncontrolled hemorrhage in four cases (5%), on account of technical difficulties in four cases (5%) such as difficult dissection for silicosis; lymph node, vascular and bronchus involvement; complex oncological cases; and stapler failure. The 87.5% of conversions were in the first year of the program. The mean surgical time was 178±65 min, in global, 197±39 min in the first year of experience, 161±61 min in the second year and 155±56 min in the third year (P value <0.005). The average time had reduced significantly along the learning curve, in a probable direct relation to the acquisition of experience and in the use of specific VATS instruments (P value <0.001) (Figure 1). We also found significant correlation between the operative time and two intraoperative findings: silicotic lymph node and incomplete or fused fissure. Comparisons between multiple variables with the outcomes are described in Table 4.
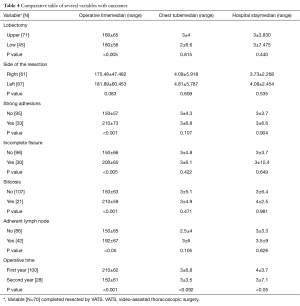
Full table
Postoperative outcomes
The median hospital stay was 3 days (range, 2–26). The median chest tube duration was 3 days (range, 0–35). The intensive care unit median time was variable during the experience (24 hours in first cases and 12 hours in later procedures).
Postoperative complications are outlined in Table 5. The most common complication was residual pleural effusion in ten patients (7.8%) followed by prolonged air leak in eight patients (6.3%) and wound infection in six patients (4.7%). In the statistical analysis we found out that; the learning curve and patients with diagnosis of COPD with FEV1 <60% and the presence of silicosis, were the only two predictive factors of complications.
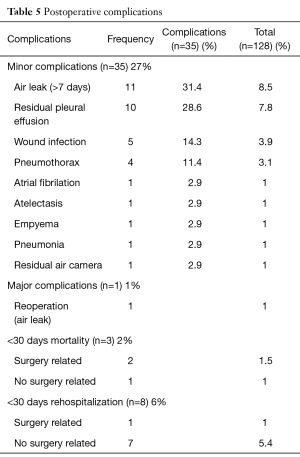
Full table
We had a major complication in one patient with heavy emphysema who required reoperation postoperatively because of a prolonged and complex air leak. The reoperation was made with the same approach without complications. There were three perioperative deaths (2.3%), two of these were associated with the procedure: (I) a right upper bilobectomy for a 5 cm long tumor in a 76-year-old man with preoperative FEV1 of 42% because of a silicosis with massive pulmonary fibrosis (the poorest pulmonary function in this study population) who died of respiratory failure on the 12th postoperative day. This patient was one of the four conversions to thoracotomy because technical difficulties; (II) a left upper lobectomy in a 76-year-old man with high cardiovascular risk because of a triple coronary by-pass, and previous right upper lobectomy with simultaneous left upper lobe wedge resection with adjuvant chemotherapy and radiotherapy treatment for synchronous lung cancer, with limited pulmonary function (preoperative FEV1: 70%) who died the day of he was discharged from hospital after an optimal recovery because of a neurological cause on the 8th postoperative day.
Complete resection (R0) was achieved in all patients. Unexpected N2 status was found in one patient. This patient was sent to the medical oncology service to complete treatment with adjuvant chemotherapy.
DiscussionOther Section
In a relatively short period of time, VATS has replaced many diagnostic and therapeutic procedures previously performed by traditional thoracotomy. Nowadays this procedure is the first choice approach for treatment of early-stage of non-small cell lung cancer (NSCLC) (16).
Over the last few years, a large number of case reviews have been published strongly suggesting that patients undergoing VATS lobectomy experience less postoperative pain, reduced chest tube time, fewer postoperative complications and shorter hospital stays than those undergoing conventional surgery. Up to now the survival rates for VATS patients appear to be similar to those reported for conventional surgery (17,18).
Roviaro et al. described the first report of VATS lobectomy in 1992 from Milan but the technique was popularized by Kirby, Landreneau, and McKenna, who after his first publication in 1994 reported the largest series of 1,100 cases in 2006 (2,19). The latest step in the evolution of the technique has been the use of a single 4 to 5 cm incision for anatomic major lung resections. This access was initially described by Rocco for diagnostic and therapeutic purposes (4) and successfully developed by Gonzalez-Rivas for lobectomies (5-7) showing good perioperative results when performed by surgeons experienced with double-port technique and anterior thoracotomy.
This study presents the initial results of the implementation of a uniportal VATS program for major lung resections performed by a single surgeon experienced in major resections by postero-lateral approach and almost all VATS procedures except major lung resections, in an area with high incidence of complex pulmonary silicosis with lymph node involvement.
Silica is the name given to a group of minerals composed of silicon and oxygen, the two most abundant elements in the earth’s crust. The three main crystalline forms are quartz, tridymite and cristobalite. Occupational exposure to breathable CS is a serious but preventable health hazard. Prolonged exposure to CS has long been known to cause one of the oldest known industrial diseases, silicosis (20,21).
Lung cancer is considered one of the serious consequences of silica exposure. In 1997, CS has been classified as a human carcinogen by the International Agency for Research on Cancer (Lyon, France). However, the working group also stated that the carcinogenicity was not found in all industrial circumstances, and the conclusion remained somewhat controversial (22).
Despite industries being aware of this disease, Spain continued record new cases of silicosis. Recent data shows that 18% of workers in the granite industry have active silicosis (23). This fact explains the high incidence of silicosis in our study sample, which was 16%.
In our study we could not establish data that directly relate silicosis with the development of lung cancer, possibly because we have two important confounders, the first: smoking, which is important potential confounding factor in the evaluation of the carcinogenicity of CS, present in 61% of our sample, and the second factor is that entire sample belongs to a region with a high incidence of silicosis.
What we have seen in our statistical analysis is that the presence of silicosis is a potential predictive factor for postoperative complications, with a P value <0.005. In the same way, we found significant prolongation of surgical time in these patients (P value <0.005). This can be justified by the technical difficulty of dissecting noble vascular structures, which are firmly attached to stony lymph nodes secondary to deposition of silica and also the trapped air within the lungs affected by silicosis cause insufficient lung collapse.
The longer oldest in uniportal VATS was published by Gozalez-Rivas in 2013 with 97 successfully major resections completed with single incision; they concluded that single-incision video-assisted thoracoscopic anatomic resection is a feasible and safe procedure with good perioperative results, especially when performed by surgeons experienced with the double-port technique and anterior thoracotomy (7).
Our experience is the second oldest experience in uniportal VATS major resections, demonstrating low complication rate and effective short-term results even under some special limitations in the training curve period: (I) a single surgeon in the department with video-thoracoscopic experience assisted by a variable inexperienced thoracic surgery resident; (II) the lack of specific instruments for VATS; and (III) inability to select cases, including complex cases such as patients with silicosis. Despite these disadvantages, our results are very similar to those described in the literature (Table 6).
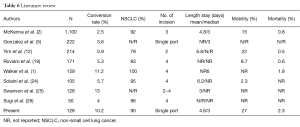
Full table
According to these results, it is recommended that an appropriate patient selection in the first part of the experience be made.
Minimally invasive techniques don’t eliminate surgical risks, and in our experience we encountered two postoperative deaths (2.3%), both in patients with an estimated higher postoperative risk. We agree with a general recommendation from experts about starting a uniportal VATS program with an initial familiarization with anterior thoracotomy and initialization in VATS major resections using at least a two ports approach before trying the uniportal technique. However our results show that a direct transition is possible from open surgery to single-port VATS for highly experienced surgeons in postero-lateral approach and other VATS procedures; even in highly complex cases (27).
Our study has several limitations. Indeed, this is a small, retrospective and descriptive study of a single-surgeon’s practice. Until there are other similar experiences, our results should be interpreted with caution. However, our study has major strengths, never before has performing uniportal VATS major lung resection in a population with a high incidence of complex cases of silicosis been written about so we believe that the description of our experience might benefit the surgical community.
In conclusion, the uniportal VATS for major anatomic lung resections is reproducible and safe with good results when performed by surgeons experienced in major open resections by anterior or postero-lateral thoracotomy, and other multiport VATS procedures even in a population area with a high incidence of silicosis. However the presence of silicosis should be taken into account as a predictive factor for postoperative complications. Therefore, overestimation of the benefits of the procedure in the patient selection process especially in the initial part of the experience must be avoided.
Despite these considerations, uniportal VATS could be considered to be the first choice to treat early-stage NSCLC; we are convinced that development of new devices and surgical instruments specifically designed for minimal access chest surgery will significantly facilitate the procedure.
Considering that our series is the second oldest performed by uniportal approach, in a subsequent study in the near future we will analyze the influence of silicosis in overall survival at 5 years in patients with lung cancer who have been operated on by this technique.
AcknowledgementsOther Section
None.
FootnoteOther Section
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional ethical review board of the Asturias University Central Hospital provided the approval for this study. Written informed consent was obtained from the patient. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
ReferencesOther Section
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Thoracoscopic lobectomy through a single incision. Multimed Man Cardiothorac Surg 2012;2012:mms007.
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- Health Effects of Occupational Exposure to Respirable Crystalline Silica. Washington, DC: National Institute for Occupational Safety and Health, 2002. Available online: http://www.cdc.gov/niosh/docs/2002-129/pdfs/2002-129.pdf
- European Commission. Brussels, Belgium: European Commission; 2006. Millions of workers’ health to be protected by Europe's first multisector agreement. Available online: http://europa.eu/rapid/press-release_IP-06-524_en.htm?locale=en, accessed June 3, 2013.
- Ministry of Health. China's Health Statistics Yearbook. Beijing: Peking Union Medical College Press, 2011. Available online: http://tongji.cnki.net/overseas/engnavi/NaviSearch.aspx?code=A&type=type&t=T
- Yim AP. VATS major pulmonary resection revisited--controversies, techniques, and results. Ann Thorac Surg 2002;74:615-23. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Aragón J, Méndez IP. First case report of single port video-assisted thoracoscopic middle lobectomy for the treatment of pulmonary aspergilloma in a pediatric patient. European J Pediatr Surg Rep 2013;1:12-4. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Maruyama R, Oka T, Anai H. Video-assisted thoracoscopic treatment for spontaneous pneumothorax as two-day surgery. Am J Surg 2000;180:171-3. [Crossref] [PubMed]
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Vida S, Pintos J, Parent ME, et al. Occupational exposure to silica and lung cancer: pooled analysis of two case-control studies in Montreal, Canada. Cancer Epidemiol Biomarkers Prev 2010;19:1602-11. [Crossref] [PubMed]
- Hessel PA, Gamble JF, Gee JB, et al. Silica, silicosis, and lung cancer: a response to a recent working group report. J Occup Environ Med 2000;42:704-20. [Crossref] [PubMed]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: International Agency for Research on Cancer, 1997;68. Available online: http://monographs.iarc.fr/ENG/Monographs/vol68/mono68.pdf
- Actualización de la lista Española de Enfermedades Profesionales. Revista del Ministerio de Trabajo y Asuntos Sociales. Available online: http://www.empleo.gob.es/es/publica/pub_electronicas/destacadas/revista/
- Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery major pulmonary resections. Present experience. Eur J Cardiothorac Surg 2001;20:437-42. [Crossref] [PubMed]
- Swanson SJ, Herndon J, D’Amico A, et al. Results of CALGB 39802: feasibility of video assisted thoracic surgery (VATS) lobectomy for early stage lung cancer. Proc ASCO 2002;21:290a.
- Sugi K, Kaneda Y, Nawata K, et al. Cost analysis for thoracoscopy: thoracoscopic wedge resection and lobectomy. Surg Today 1998;28:41-5. [Crossref] [PubMed]
- Aragón J, Pérez Méndez I. From open surgery to uniportal VATS: asturias experience. J Thorac Dis 2014;6:S644-9. [PubMed]
Cite this article as: Aragón J, Pérez Méndez I. Four years’ experience in uniportal video-assisted thoracoscopic surgery for major lung resections: influence of silicosis in clinical outcomes. J Vis Surg 2016;2:95.