
The technical details of robotic-assisted mitral valve replacement
Introduction
Valve replacement is an important treatment method for severe mitral valve pathologies when repair is not feasible. Technological developments have increased the types and the number of minimally invasive interventions for mitral valve pathologies (1). The surgical trauma of the patient is minimized and a better surgical exposure is provided with the robotic approach. During the robotic-assisted surgery, the tip of robotic instruments can rotate up to 540 degrees of motion which provides better dexterity than human wrist.
Additionally, those instruments are smaller than surgeon hands which allows to reach surgical points where surgeon hands cannot be able to. Robotic endoscope provides a very close approach to the left atrium and mitral valve apparatus thanks to its dexterity, 3D visualization and zooming capabilities. The robotic left atrial retractor assists the surgeon not only in left atrium but also at many points due to its multifunctionality and maneuverability such as while placing the pacewire, suturing the aortic hole, closure of patent foramen ovale (PFO), performing cryoablation or repairing the tricuspid valve. Thanks to this feature, the robotic left atrial retractor is distinctly different and useful from the retractors during the conventional minimally invasive surgery. All these factors may assure a more effective and safer mitral valve repair/replacement with robotic surgery.
In this section, the steps of robotic assisted mitral valve replacement will be detailed and enriched with 10 video images. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this study and any accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Preoperative evaluation
After the transthoracic echocardiography, patients who are over 40 years old and/or have risk factors for atherosclerosis should be evaluated with coronary angiography. If renal functions are in normal range, a contrast enhanced tomography would detail the entire major vascular system, including the common femoral arteries. This examination may guide the appropriate surgical strategy, such as the cannulation site, use of antegrade vs. retrograde flow and whether an aortic cross clamp should be applied. The patients with renal problems are routinely consulted with a nephrologist and the aforementioned imaging can be performed without contrast.
Contraindications for robotic mitral surgery may include severe coronary artery disease requiring bypass surgery, presence of extensive atherosclerosis including the axillary artery preventing safe arterial cannulation and redo operations that have caused very serious pleural adhesions.
Surgical technique
The operating room should be large enough to place all relevant devices including patient side and vision carts, the surgeon console, multiple monitors, transesophageal echocardiography (TEE) device, cardiopulmonary bypass (CPB) machine, and heat exchanger for robotic-assisted cardiac surgery (Video 1). After induction of general anesthesia, intubation is achieved via a double-lumen endobronchial tube and then a TEE probe is placed. Cerebral oxygenation is followed via cerebral oximeter throughout the operation. Under the right shoulder, a medium sized chest roll is placed, the right shoulder remains at the edge of the operating table and the table is lifted to the 15 degrees right side up position. The port entry and incision sites are marked (Video 2).
Peripheral cannulation
Following the central venous cannulation and 5,000 Units of heparin administration a 15- or 17-French (F) venous cannula is placed via the right internal jugular vein to the vena cava superior, with its tip being 1 cm inferior to the cava-atrial junction (Video 3). All these steps are performed under ultrasound (USG) and TEE guidance respectively. The liberal usage of USG and TEE at every stage of operation minimizes the complications and provides a comfortable operation.
Ultrasonographic evaluation of the femoral artery and the vein provides to avoid cannulation of the calcified portions of artery and to select an appropriate cannula size. Selecting the suitable cannula size is crucial to maintain effective CPB circulation especially in obese patients. Cannulation of axillary artery is a better strategy to avoid neurological injury in patients with highly calcification.
Arterial and venous cannulation may be performed via percutaneous or open technique after achieving the target activated clotting time (ACT) (>400 s) (350 U/kg of heparin) (Videos 4,5). The percutaneous technique under USG guidance may be helpful to avoid wound-related complications. For this technique, we use the Perclose ProGlide closure device (Abbott Vascular, CA, USA). The technical details with clinical results were reported previously (2) (Video 4). But briefly, under USG guidance the common femoral artery is punctured with a 5-F needle then a 6-F sheath is placed. The 0.035 guidewire is advanced over the sheath, then the sheath is removed, leaving only guidewire in the femoral artery. The accurate placement of this 0.035 guidewire is checked with TEE. From the safety side, two ProGlide sutures are necessary to deploy one at the 10 o’clock and the other at 2 o’clock positions on the femoral artery. The sutures are released from the device and clamped with no tightening the knots. After the sutures are released from the device, the guidewire for the arterial cannula is then advanced again under TEE guidance. Visualization of the guidewire in the descending aorta with TEE is required prior to cannula insertion through the artery. The puncture site is dilated with appropriately increasing sized dilatators for the placement of 15–22-F arterial cannula. Small sized arterial cannula may cause increased shear stress and hemolysis, especially during long procedures. Insertion of a second cannula from the contralateral site may be necessary to avoid increased pressure at lines of CPB.
The femoral venous cannula can be inserted percutaneously from the ipsilateral or contralateral site, similar to jugular vein cannulation. TEE confirms that the venous cannula is at the cava-atrial junction (Video 5). The tip of the cannula at the SVC, is positioned 1 cm superior to the cava-atrial junction, and the tip of the cannula at the IVC is placed 1 cm inferior to the cava-atrial junction during concomitant tricuspid surgery. This technique provides enough space for clamping the venae cavae before opening the right atrium.
Port placement
Port placement from the appropriate position is one of the critical steps during the operation (Video 6). Otherwise, narrow working space in the right chest or the collision of the robotic arms may cause technical challenges during the surgery. This problem has been largely solved by the new version of Da Vinci’s Xi systems which determines the target point (working port) via the laser.
A 4 cm incision is made for a working port on the 4th intercostal space, 1–2 cm medial to the anterior axillary line. Care should be taken not to damage the breast tissue in women. A 30-degree endoscope is inserted via this port for the security reasons and all port placements are guided by this endoscope. The port for the right arm is placed at the 5th or 6th intercostal space on the anterior axillary line caudal to the working/camera port with keeping a 6–7 cm distance. The port for the left arm is created at the 3rd intercostal space and 2–3 cm medial to the anterior axillary line, keeping a 6–7 cm distance with the other ports. One more small incision is made in the 2nd or 3rd intercostal space on the mid-axillary line to insert the aortic clamp (Chitwood clamp) and left atrial sump. Finally, the port for left atrial retractor is placed 4–5 cm medial to the working port parasternally, taking care not to injure the right internal thoracic artery. A small size soft tissue retractor is placed in the working port instead of a rib spreader to reduce postoperative pain. The camera is placed through the soft tissue retractor. Carbon dioxide is applied with 8-mmHg pressure and a flow rate of 6 L/min. After the implantation of the ports, the robotic arms are connected to the ports (docking) in the next step. The center column of the robot should be lined up with the camera port. Once the docking is completed, the operating table should not be moved at any time. At this time, the surgeon can take responsibility for the operating of the robot. The following instruments are used for right or left robotic arms during daVinci Robotic Assisted Systems: Debakey forceps, monopolar curved scissors, large needle driver and atrial retractor.
CPB, cross clamping and myocardial protection
CPB is initiated following the target ACT level is reached and completion of the cannulation. If venous return is not sufficient, venous suction can be used, taking care not to exceed 45–50 mmHg. When there is a problem with venous return even with the suction, the tips of venous cannulae are checked again with TEE. The pericardium is opened at least 2–3 cm. far above from the phrenic nerve. Two pericardial and a diaphragm stay sutures are snared and pulled through the chest wall then fixed externally for a better exposure. The ascending aorta is clamped via Chitwood clamp, which is inserted through the second intercostal space in the direction of the transverse sinus. In this step, care should be taken not to injure the left atrial appendage or the pulmonary artery.
Using endoaortic balloon is another technique to achieve aortic occlusion. This is achieved with a balloon tipped catheter which is inserted either from the femoral artery or directly through the ascending aorta. The localization and inflation of balloon is guided by TEE and there is a necessity of close monitoring of the balloon which may displace during the procedure. Therefore we prefer using Chitwood clamp which is simpler to apply and cheaper.
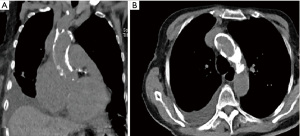
Single-dose cold (5 ℃) Del-Nido cardioplegia is delivered into the aortic root via the service port to arrest the heart and should be confirmed with TEE guidance (Video 7). The insertion point of the cardioplegia needle (16 G) on the ascending aorta may be marked to avoid suturing an improper point later on. Avoiding to clamp the aorta may be a safer approach in patients with highly aortic calcification or adhesion at mediastinum (Figure 1). The surgery can be continued with fibrillation of the heart via fibrillator electrodes in this situation. The left ventricular distention and air embolism are the important issues should be taken into consideration. These can be prevented by carbon dioxide insufflation and placing two sumps, one into the left atrium and the other into the left ventricle via the mitral valve (3).
Atriotomy and exposure of the mitral valve
The interatrial groove approach is used for the left atrial exposure. The incision may be extended inferiorly along the midline between the right inferior pulmonary vein and the inferior vena cava. The mitral valve is exposed after placing the left atrial retractor (Video 8). The transseptal approach for the exposure of mitral valve is not recommended due to the short distance between the septal incision and the anterior mitral annulus. This may prevent to place the left atrial retractor safely.
Valve replacement
In the presence of atrial fibrillation, the left atrial appendage should be closed with a 4-0 polypropylene suture. When ablation is planned, the procedure can be performed with a radiofrequency or cryoablation probe (Video 9).
In rheumatic disease, the leaflets are non-pliable and thickened with calcifications of varying degrees in most of the cases. Subvalvular structures may also be thickened and retracted. In such cases, the leaflets are grabbed with a grasper and excised with scissors. Alternatively, the cutting mode of electrocautery may be used for severely fibrotic or calcified tissues when the robotic scissors do not cut efficiently (Video 10). The subvalvular tissue should be preserved as much as possible; however, the resection of this structure is inevitable if the disease is widespread and severe. Valve sizing can be done by inserting the appropriate sizer into the left atrium. Also, the excised valve can be measured at outside of the thoracic cavity. After valve resection, all the 2-0 polyester pledgeted sutures (generally 12–14 sutures) are passed through the annulus by the console surgeon. Sometimes suture management can be confusing. To overcome this situation two different colored sutures are used in a systematic order and placed to the suture organizer again systematically. After completing the suture placement at the mitral annulus then all sutures are passed through the sewing ring of the prosthesis by the assistant surgeon. The prosthesis is glided down into the native annulus and the valve handle is released. The holder is removed, turned the sideway of prosthesis and inserted in patients with narrow interspaces. If there is still difficulty to insert, the intercostal space is retracted via the Farabeuf retractor then the prosthesis can be inserted.
The sutures are knotted by the assistant surgeon, either using a knot-pusher or a Cor-Knot device (The Cor-KnotTM, LSI Solutions, Inc., Victor, NY, USA). The latter option uses a titanium clip to secure sutures and saves up to 10 minutes per prosthesis (Video 10).
Atriotomy closure
The atriotomy may be closed using two premade 3.0 prolene loop sutures (Leyla Loop) which saves an additional time of around 5 minutes (4). A sump is inserted through the atriotomy incision. A purse suture with a 4-0 polypropylene is placed to the previously marked point on the ascending aorta for the venting cannula. After de-airing via venting cannula, the cross-clamp is removed. The implanted valve is checked using TEE. The lead of pacemaker should be placed before weaning of CPB. The needle of lead can exit from the right port with the help of endoscopic camera.
Weaning from CPB, decannulation and last steps
If the patient is hemodynamically stable and prosthetic valve functions normal, weaning from CPB can be initiated. Decannulation starts with venous cannulas and the blood in venous lines can be stored at reservoir. There is no need to use a vascular closure device after decannulation of the venous cannula in percutaneous technique. However, a purse or Z suture with compresing with sandbag is necessary for bleeding control. If the open technique is used, the vein is repaired with a previously placed 5.0 prolene suture after the venous cannula is removed.
Arterial closure is performed according to the characteristics of the device when the percutaneous technique is used. For the Perclose ProGlide systems, the two knots are tied with knot pusher after the femoral decannulation. In the open technique, the knot is tied via previously placed 5.0 prolene purse suture after the arterial cannula is removed.
After decannulation, heparin is reversed and hemostasis is achieved with the help of endoscopic camera. The endoscope can be placed from the different ports to inspect all different surgical areas which has possibility of bleeding. Hemostasis is another very important step of robotic surgery. Revision for bleeding especially in urgent set-up would be highly difficult to manage and may need to enlarge the incision of thoracotomy.
After bleeding control all incisions are closed in layers. The robotic arms are removed and the drainage tube is placed through the right port incision.
Comments
The adoption of robotic technique is more slowly in cardiac surgery comparing to other specialities for many reasons, including cost, the need for dedicated anesthesiologists, nurses, perfusionists, and more importantly, lack of proper training of whole team (5). In a recently published study, we analyzed the learning curve for mitral valve repair, which may be applicable to replacement procedures as well (6). In that study, we analyzed and compared the CPB and CC times as the measurement tools for the learning curve and had encouraging results for the surgeons who desire to start a robotic mitral surgery program. After approximately 30 cases, CPB and CC times were no longer significantly different from those observed for conventional mitral surgery. Currently CC and CPB times for isolated robot assisted mitral valve replacement are 50–60 and 70–80 minutes, respectively. Early results of our another study showed no perioperative mortality and coequal or superior outcomes compared to the conventional technique in terms of bleeding, infection, blood transfusion and length of stay in intensive care unit (ICU) (7).
Mitral valve diseases are generally associated with other pathologies such as tricuspid valve insufficiency and atrial fibrillation. In such cases, robotic assisted intervention for the tricuspid valve, cryo- or radiofrequency ablation and left atrial appendage ligation in addition to mitral valve replacement are all feasible without significant postoperative mortality and morbidity (7).
Innovations of technology companies provide solutions for the important problems encountered during robotic mitral valve operations. The problem of robotic arm collision in a narrow space has been eliminated by the innovation of laser target point in the new version of robotic system (XI systems). In this model, another characteristic is the improved movement of arm angles which enables more practical use for heart surgery. Moreover, the camera can be used by the different ports and this provides an important advantage during bleeding control.
Another way to insert the mitral valve is to use transcatheter techniques; however, achieving a fully percutaneous mitral valve intervention is a more complicated procedure than transcatheter aortic valve implantation (TAVI) (8). The left ventricular size, aortomitral annular angle, and the degree of septal hypertrophy are all challenging factors when planning during transcatheter procedures. The use of the robotic approach in such complex situations allows the patient to be treated minimally invasively and provides the surgeon to repair with direct vision.
In conclusion, the robotic approach to the mitral valve provides perfect surgical exposure and consequently safe valve replacement. Thanks to the increased maneuverability of the robotic arms and retractors, concomitant procedures such as cryoablation, PFO or atrial septal defect (ASD) closure, ligation of left atrial appendage and intervention for tricuspid valve can be performed after a learning period.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Johannes Bonatti) for the series “Robotic Mitral Valve Repair” published in Journal of Visualized Surgery. The article has undergone external peer review.
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-22-35/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-22-35/coif). The series “Robotic Mitral Valve Repair” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Palmen M, Navarra E, Bonatti J, et al. Current state of the art and recommendations in robotic mitral valve surgery. Interact Cardiovasc Thorac Surg 2022;35:ivac160. [Crossref] [PubMed]
- Bastopcu M, Senay S, Güllü AÜ, et al. Percutaneous cannulation for cardiopulmonary bypass in robotic mitral valve surgery with zero groin complications. J Card Surg 2022;37:280-4. [Crossref] [PubMed]
- Alhan C, Şenay Ş, Koçyiğit M, et al. Robot-assisted mitral valve surgery without aortic cross-clamping: An alternative technique. Turk Gogus Kalp Damar Cerrahisi Derg 2021;29:415-6. [Crossref] [PubMed]
- Kiliç L, Şenay Ş, Ümit Güllü A, et al. Leyla loop: a time-saving suture technique for robotic atrial closure. Interact Cardiovasc Thorac Surg 2013;17:579-80. [Crossref] [PubMed]
- Rodriguez E, Nifong LW, Bonatti J, et al. Pathway for surgeons and programs to establish and maintain a successful robot-assisted adult cardiac surgery program. J Thorac Cardiovasc Surg 2016;152:9-13. [Crossref] [PubMed]
- Güllü AÜ, Senay S, Kocyigit M, et al. An analysis of the learning curve for robotic-assisted mitral valve repair. J Card Surg 2021;36:624-8. [Crossref] [PubMed]
- Güllü AÜ, Şenay Ş, Koçyiğit M, et al. The feasibility of robotic-assisted concomitant procedures during mitral valve operations. Turk Gogus Kalp Damar Cerrahisi Derg 2019;27:478-83. [Crossref] [PubMed]
- Fiorilli PN, Herrmann HC, Szeto WY. Transcatheter mitral valve replacement: latest advances and future directions. Ann Cardiothorac Surg 2021;10:85-95. [Crossref] [PubMed]
Cite this article as: Gullu AU, Senay S, Ersin E, Kocyigit M, Kılıç L, Celik O, Alhan C. The technical details of robotic-assisted mitral valve replacement. J Vis Surg 2023;9:22.


