Robotically assisted mitral valve repair—resectional techniques
Introduction
Robotic mitral valve repair was introduced as a minimally invasive method in 1998 by Carpentier in Paris (1) and has since developed into a reproducible and mature procedure with competitive results (2). Essentially all repair techniques that are applied in open mitral surgery through sternotomy can be performed robotically. Robotics enables a more complex repair than minimally invasive mitral valve repair using standard videoscopy and long-shafted thoracoscopic instrumentation (3). To repair the posterior leaflet of the mitral valve, resection of flail and prolapsing segments or chordal replacement with artificial chords are performed in open, minimally invasive, and robotic practice. There is an ongoing debate about which method is better. In prospective randomized trials, however, no significant clinical advantage has been demonstrated either way (4,5).
Aim of the video and technical paper
This technical video (Video 1) and paper describe our robotic mitral valve repair technique using an adjunct mini-thoracotomy and leaflet resection to treat posterior leaflet prolapse and flail posterior leaflet. The advantages and challenges of these techniques are highlighted.
All procedures performed for this publication were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this article, the accompanying video, and images.
Surgical techniques
Patient selection
Any patient that qualifies for surgical mitral valve repair can be considered for a robotic approach. In addition to the usual preoperative tests for open cardiac surgery, patients undergo a computed tomography (CT) angiography of the chest, abdomen, and pelvis, as well as pulmonary function tests. Classic exclusion criteria are severe mitral annular calcification, endocarditis, greater than trace to mild aortic valve regurgitation, ascending aortic dilatation greater than 4 cm, severely impaired left ventricular function, and pulmonary hypertension. Other contraindications are significantly impaired pulmonary function, previous cardiac or ipsilateral thoracic surgery, chest deformities, morbid obesity, and severe atherosclerosis on the aortoiliac level. Advanced robotic mitral valve repair programs, however, have carefully challenged these contraindications and published successful experiences using the robot for the abovementioned pathologies (2).
Anesthesia management
Patients receive a paravertebral block for intra- and postoperative regional anesthesia in the operating room (OR) prep area. In the OR, general anesthesia is induced, and either a double-lumen endotracheal tube or a single-lumen tube and a bronchial blocker are used to isolate the right lung. In addition to usual monitoring and infusion lines, percutaneous defibrillator pads are placed. It is crucial to ensure adequate electric current flow through the latter. Therefore, one pad is placed under the right shoulder, and the counter-pad is placed on the left anterior chest wall. Near infrared spectroscopy (NIRS) monitoring of the brain and both legs is conducted throughout the procedure to ensure adequate end-organ perfusion. The anesthesiologist inserts a percutaneous 15- or 17-Fr venous drainage cannula into the right internal jugular vein. The distal tip is guided just above the cavoatrial junction and confirmed by echocardiography. As the patient is not heparinized at this point, continuous infusion of heparin into this cannula is essential. The cardiac surgeon is advised to carry out a final review of the mitral valve pathology on transesophageal echocardiography (TEE) with the anesthesia and cardiac surgery teams before the start of the operation. Specific attention should be paid to identifying systolic anterior motion (SAM) risk factors.
Robot position and draping of the robotic arms
We currently use the da Vinci Xi robotic system (Intuitive Sunnyvale, CA, USA). The patient cart is placed on the patient’s left side, and the arms reach the right chest. The robotic console is ideally positioned on the patient’s right side to allow for optimal communication between the console surgeon and the bedside team. At University of Pittsburgh Medical Center (UPMC), we have an additional console available for teaching purposes and for performing complex cases by two experienced robotic surgeons.
Patient positioning, prepping, draping, and preparation for the start of the procedure
The patient is positioned supine. A sandbag or towel roll is placed underneath the right chest to elevate it by approximately 30 degrees. Both arms are padded, tucked, and secured with table towels. It is essential to place the right chest as close as possible to the right edge of the operating table to ensure good access to the chest wall. The patient’s chest is prepped and draped for an open cardiac case; in case an emergent sternotomy is required at any time during the procedure. Both groins and thighs are exposed as well. Leaving the axillary fold and the area behind the anterior axillary line undraped on the right chest is important because the left atrial suction and the transthoracic clamp will enter these regions.
To smoothly conduct the procedure, all cardiopulmonary bypass lines, cables, and devices necessary for the operation are brought onto the operative field. Once the instruments are in position, a timeout is conducted.
Mini-thoracotomy and port placement
At the time of the incision, the right lung is collapsed. A right mini-thoracotomy incision is made to access the mitral valve. For placement, the surgeon places the right third fingertip on the xiphoid angle and the left third fingertip on the sternal notch. A triangle is formed with both hands, and the entry point is defined as where the two thumbs meet. This is usually the middle of the right chest, in the 4th intercostal space, approximately two finger widths anterior to the anterior axillary line. The intercostal space is palpated, and the incision follows the trajectory of the interspace direction towards the xiphoid angle. This method is described in the attached video (minute 0:29 onwards). The mean length of our mini thoracotomies is 6.4±1.2 cm. Alternatively, a periareolar approach can be chosen (6).
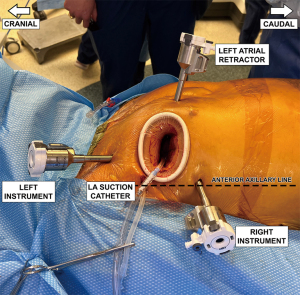
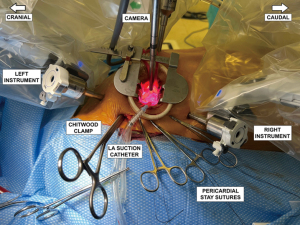
After careful hemostatic dissection through the subcutis and thoracic musculature, the interspace is entered, and an Alexis small soft tissue retractor is inserted. An additional small metal retractor can be helpful. The robotic instrument ports are inserted two interspaces off the mini-incision, the right on the anterior axillary line and the left three-finger widths anterior to the anterior axillary line. Ports are inserted under finger guidance and the port tips should be visible through the mini-thoracotomy. The left atrial retractor port is placed on the sternal side of the mini-thoracotomy. The surgeon makes sure not to go too close to the internal mammary artery, which may also be palpated. The final port arrangement is shown in Figure 1. We utilize the mini-thoracotomy as the “camera port’ which avoids the need to create a separate incision for the camera. The transthoracic aortic cross-clamp and the left atrial suction tube are inserted through a separate small incision in the axillary fold. For the left atrial suction tube, we suggest protecting the tip during insertion with a piece of heart-lung machine suction tubing. This is because subcutaneous fat may accumulate in the suction tip and lead to arterial embolization.
Pericardial fat pad removal and robot docking
The pericardial fat pad is removed using standard minimally invasive cardiac surgery (MICS) instrumentation. Care is taken not to injure the innominate vein and the phrenic nerve. During this maneuver, the robot is prepared for docking.
The robotic patient cart is moved toward the patient’s right chest using laser targeting, where the laser cross should meet the sternal part of the mini-thoracotomy. The arms are then docked to the ports. The surgical team ensures enough distance between the arms to avoid collisions. The left instrument arm (arm number 1 on the da Vinci Xi system) is docked first, followed by the atrial retractor arm (arm number 3 on the da Vinci Xi system). Then a port is mounted on the camera arm (arm number 2 on the da Vinci Xi system), and the robotic three-dimensional (3D)/high-definition (HD) camera is inserted. The camera view is always 30 degrees up in robotic mitral surgery cases. The camera is then placed into the sternal part of the mini-thoracotomy to ensure additional lighting of the thoracic cavity. We leave arm number 4 on the da Vinci Xi system undocked at this point because further maneuvers of the surgeon’s right arm are necessary that can be compromised by the robotic arm. The port arrangement with da Vinci Xi arm numbers 1–3 docked to the ports is shown in Figure 2.
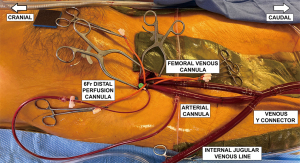
Installation of peripheral cardiopulmonary bypass
In parallel to the abovementioned steps, another surgical team member carries out peripheral cannulation. This strategy can save operative time. A right groin incision is made, and the anterior walls of the femoral artery and vein are exposed. We only expose the anterior wall of the vessels. Going around them increases the risk of lymphatic fistulas. The patient is fully heparinized, and 5-0 Prolene purse string sutures and tourniquets are used to secure all cannulae. A 6-Fr wire-reinforced perfusion cannula is inserted into the right superficial femoral artery to ensure adequate leg perfusion.
To achieve adequate visualization of the left atrium and the mitral valve, we prefer using bicaval drainage. In addition to the internal jugular venous cannula, a multistage cannula is inserted into the femoral vein using the Seldinger technique. For adequate guide wire placement into the superior vena cava, the anesthesiologist presents a bicaval view on TEE. With this arrangement, the J of the wire can be appreciated as it enters the right atrium and, subsequently, the superior vena cava. Any arrhythmias should be carefully noticed as they may indicate the guidewire entering the right atrium or ventricle. Having confirmed the proper guidewire position, the cannula is advanced into the superior vena cava. After removal of the trocar, the cannula is connected to the heart-lung machine tubing. At this point, the superior vena cava cannula is also connected to the heart-lung machine tubing. Next, a 17- or 19-Fr arterial perfusion cannula is inserted into the right common femoral artery. It is advanced after visualization of the guide wire in the descending thoracic aorta. The final femoral cannulation arrangement is shown in Figure 3.
Following the connection of all cannulae, retrograde arterial and venous priming maneuvers are carried out. Before starting the heart-lung machine, the descending thoracic aorta is visualized on TEE. The whole team of surgeons, anesthesiologists, and perfusionists watches the descending thoracic aorta during the start of perfusion. This is because, in the rare but catastrophic case of a retrograde aortic dissection, the development of the dissection flap can be observed, and the pump can be stopped immediately.
Pericardiotomy and induction of cardioplegic arrest
With the patient on pump, the pericardiotomy is straightforward as the heart is unloaded, and enough space between the pericardial sac and the cardiac structures is created. The longitudinal pericardiotomy is carried out using standard MICS instrumentation and cautery. It is important to stay away at least 2 cm from the phrenic nerve. Three pericardial stay sutures are then placed. We start with the most caudal one and catch it with an EndocloseTM device after a transthoracic puncture below the right instrument port. If the diaphragm is high, it can be pulled caudally using this retraction stitch as an alternative to a diaphragmatic traction suture. The two other pericardial stay sutures are placed in the middle and cranial parts of the pericardiotomy. Stay sutures are tightened and secured on the thoracic wall using clamps as it may be necessary to loosen them during the later conduct of the procedure.
A 3-0 Prolene pledgeted stitch is placed on the ascending aorta around the transverse fat streak. A long cardioplegia cannula is inserted into the ascending aorta and secured with a tourniquet. The cannula is connected to the cardioplegia and vent lines using a Y-connector and the cardioplegia line is de-aired.
We then bring the transthoracic Chitwood clamp into position, ensuring it is adequately away from the left atrial appendage and the right pulmonary artery, as injuries to both structures can occur. The aorta is then cross-clamped, and cardioplegia is infused. We currently use del Nido cardioplegia following standard protocols. The patient is cooled to 32 ℃.
Left atriotomy and mitral valve inspection
During induction of cardioplegia, the right robotic arm is finally docked. Robotic instruments are inserted into their corresponding ports: left atrial retractor into the retractor port, robotic DeBakey forceps into the left arm, and a large robotic needle driver into the right arm ports. The patient-side surgeon, under direct vision, advances the instruments. The port arrangement with all four robot arms docked, with the Chitwood clamp and retraction sutures held by hemostats, are shown in Figure 4. The primary surgeon scrubs out and starts work at the console.
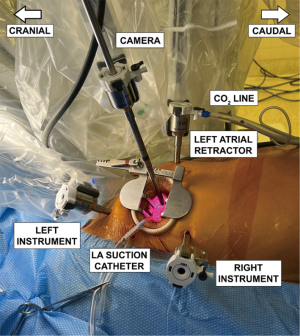
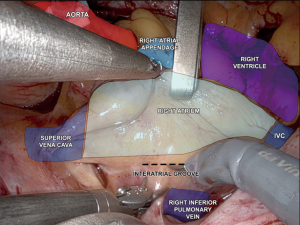
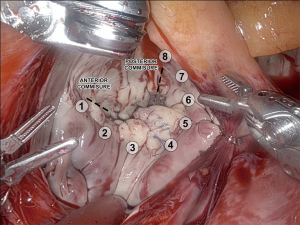
We first expose the oblique sinus using blunt dissection with the robotic de Bakey forceps and the large needle driver. This maneuver enhances the robotic view of the left atrial structures. The view of the pertinent intrathoracic anatomy prior to the left atrial incision is shown in Figure 5. The left atrium is incised with curved robotic scissors at the junction between the right superior pulmonary vein and the left atrium, which is suctioned out by the patient-side surgeon. The left atrial retractor is inserted, and the incision is carried cranially and caudally. The left atrial suction catheter is inserted into the left-sided pulmonary veins and tacked to the cranial pericardial edge. A 3-0 Prolene stitch is placed into the left atrial wall close to the P3 area of the mitral valve and tacked to the pericardium adjacent to the inferior vena cava. Both tacking sutures are secured with a CorKnotTM.
A usual valve inspection is carried out, assessing all valve segments. A water test to identify prolapsing or flail segments may be added.
Posterior leaflet repair using resectional techniques
Triangular resection
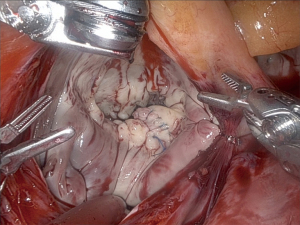
The most common resection technique we perform is a triangular resection of the P2 segment. The tip of P2 is grasped with the robotic de Bakey forceps on the left instrument arm and pulled up. The posterior leaflet is inspected for adjacent reasonably healthy chords, and a resection line is projected. We also use a ruler brought in by the assisting surgeon to measure the length of the reconstructed leaflet after resection, aiming for approximately 15 mm from the annulus to the tip of the leaflet. Monopolar curved robotic scissors are used. The triangular resection starts to the right of the tip of P2 and aims at the center of P2 on the annulus. It is then carried towards the free edge of the leaflet to the left of the tip of P2. Resected primary or secondary chords are detached from the papillary muscles. The resected leaflet segment is handed over to the assisting surgeon, who takes it out of the thoracic cavity using a MICS grasper. Figure 6 shows the mitral valve after triangular resection.
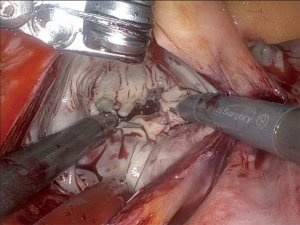
The defect, after resection, is closed using a double layer running 4-0 Prolene suture using a large needle driver on the right robotic arm and a de Bakey forceps on the left robotic arm. The patient-side surgeon hands the suture over to the console surgeon, who places the first stitch on the leaflet tip. The patient-side surgeon then takes it out and pulls half of the suture to the outside of the chest. The needle is then handed back to the console surgeon. For each stitch of the running suture, the patient-side surgeon, after needle passing, catches the thread adjacent to the needle with a nerve hook and pulls it tight. During this maneuver, the console surgeon gets the needle into position and then places the next stitch. This “hooking” version of a running endoscopic suture is very efficient. After completing the second layer, the suture is tied using a knot pusher. Six knots are applied. Figure 7 shows the mitral valve after repair of the triangular resection defect using double layer suture technique.
Quadrangular resection and sliding plasty
A quadrangular resection may be carried out in cases of abundant leaflet tissue. The leaflet remnants need to be mobilized as direct suture of the defect would lead to excessive suture tension. Using curved scissors, they are incised at the annulus and cut off the annulus up to an area close to the commissures. We then unite the leaflet remnants at the annulus with a double-armed 4-0 Prolene suture and stitch the needles through the former center of P2 on the annulus. This suture, at this point is not tied. The leaflet remnants are tacked to the annulus using several single interrupted 4-0 Prolene sutures, each 8 cm in length. They are tied with robotic instruments. Lastly, a double layer 4-0 Prolene running suture is carried from the tip of the “neo P2” segment to its base at the annulus and tied to the first suture in this process.
As an alternative to the sliding plasty, a folding plasty can be carried out in which the leaflet remnants after quadrangular resection are folded towards the former center of P2 and tacked to the annulus using single interrupted 4-0 Prolene sutures. A longitudinal double layer running 4-0 Prolene from the tip of the “neo P2” segment to its base at the annulus follows.
After the leaflet reconstruction, we usually conduct a water test to assess the primary repair result.
Annuloplasty
Resectional repair of the posterior leaflet is usually followed by an annuloplasty using a partial flexible band. The industry now offers sizers that are appropriate for endoscopic use. Sizing follows the general principles of mitral valve repair.
The location of the eight annuloplasty stitches on the mitral annulus is shown in Figure 8. While the band is prepared, the console surgeon places one or two annuloplasty stitches close to the left mitral trigone. These are brought through the band by the patient side team outside the chest. The band is then lowered to the annulus. The console surgeon places the next annuloplasty stitch through the band. While this is done, the patient-side surgeon anchors the first stitch at the left trigone with a CorknotTM. The third stitch is then completed at the annulus and brought through the band. Anchoring the second last annuloplasty stitches with CorknotTM before placement of the next one ensures that the endoscopic operative field does not become crowded with stitches and enables a high comfort level during the annuloplasty. Depending on the band size, we usually place eight to ten stitches. A water test is carried out to assess the repair result. Clefts may be closed with single interrupted 8cm 4-0 Prolene sutures or a running 4-0 Prolene suture.
Atriotomy closure
The left atrial retractor is adjusted to optimize the view of the atriotomy. In most cases, we start closing it from the cranial edge because suturing is more challenging in this region. A CV 4 GoreTex suture is used. A so-called “Leyla loop” is tied at the end of the suture (7). After passing the first stitch, the needle is brought through the loop, and the suture is pulled tight. This elegantly saves one intrathoracic knot-tying maneuver. The running suture is carried out using a nerve hook to pull the sutures tight. After reaching half of the atriotomy, another suture is started at the caudal edge. Before closure, the left atrial suction catheter and the left atrial retractor are pulled out. The two sutures are tied together using a knot pusher.
Pacemaker wire placement, de-airing, and reperfusion
It is crucial to place a temporary pacing wire while the heart is still arrested. Later, this maneuver is difficult or even impossible. Lifting up the pericardium with the left atrial retractor helps with exposure. The acute margin of the heart is gently mobilized with DeBakey forceps, and the needle of the pacing wire is brought through the right ventricular myocardium. We place one such wire on the heart. The grounding wire goes through the skin close to the mini-thoracotomy.
The heart is then de-aired through the aortic root vent cannula, the heart is filled, and the anesthesiologist manually inflates the left lung. Then the transthoracic aortic cross-clamp is released. Lung inflations are stopped, and the heart is unloaded again. Checking for hemostasis on the heart follows. We carefully review the atriotomy, and preemptive 3-0 pledgeted Prolene repair stitches are placed at any suspicious sites. The left atrial appendage and the right pulmonary artery are meticulously inspected for bleeding. Robotic endoscopic repair stitches are possible at these sites while the patient is still on pump. They are much more difficult at a later time point.
Weaning off cardiopulmonary bypass, assessment of the repair result, and decannulation
Residual blood is aspirated from the right pleural space. Both lungs are then inflated. The pericardial stay sutures need to be loosened for inflation of the right lung. The heart is filled, and the patient is weaned from cardiopulmonary bypass. Once off the heart-lung machine, surgeons and anesthesiologists check the repair result on TEE. We carefully assess residual valvular regurgitation, transvalvular gradient, and SAM of the mitral valve. If the result is satisfactory, the robot is undocked.
The cardioplegia/vent cannula is removed from the aortic root while the patient is still cannulated. The pledgeted suture can safely be secured with a Corknot rather than a knot pusher. Then groin decannulation is carried out, and protamine is given.
Hemostasis and last steps
After removal of the soft tissue retractor, the thoracic cavity is thoroughly inspected for bleeding using direct vision and a small thoracoscope. The right lung must be collapsed for inspection to take place. As some patients develop oxygen desaturations during this phase, intermittent double lung ventilation may be necessary. The thoracoscope is inserted through the robotic ports, which are kept in place while waiting on the effect of protamine. As the atrial retractor port is the least likely to bleed, we first pull this one back and inspect the porthole from the left instrument port or through the mini-thoracotomy. The port is reinserted, and we concentrate on videoscopic assessment of the left instrument port. As the pectoralis major muscle is involved in the port trajectory, bleeding is likely. The hole is cauterized under scope vision with the extended electrocautery spatula inserted through the mini-thoracotomy. If oozing cannot be controlled, we pack the porthole with Surgicel. The insertion site for the Chitwood clamp is usually larger and can be inspected directly after the insertion of a sponge stick holder. Cauterization is carried out under direct vision. Thoracoscopic inspection is also carried out through the left atrial retractor port. The same applies to the right instrument port, where hemostatic control is important as the chest tube is inserted here. We usually insert a 24-Fr Blake drain. The edges of the mini-thoracotomy are finally inspected directly and with the scope. Local anesthetic is injected into the mini-thoracotomy interspace, and two pericostal sutures are placed. Before final closure, right lung recruitment maneuvers are undertaken under direct vision.
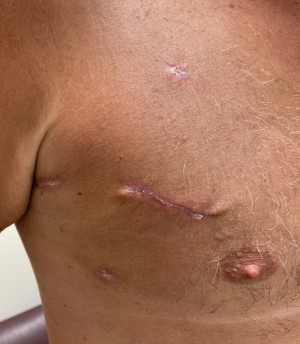
The procedure is completed with the closure of all ports, the thoracic mini-incision, and the groin incision. Extubation in the OR is possible in most cases. Figure 9 shows the healed robotic mitral valve repair incisions at a 4-week postoperative visit.
Comments
Main advantages of resectional techniques in robotic mitral valve repair
The multi-wristed robotic end effectors enable comfortable resection maneuvers, which are more challenging with long-shafted MICS instruments, specifically if advanced techniques such as sliding plasty are necessary. The same applies to suturing the defects after leaflet resection. The 3D/HD view with the robotic camera provides total virtual immersion of the robotic surgeon to the front of the mitral valve, and spatial orientation is enhanced compared to 2D videoscopic approaches. In addition, the robot corrects surgeon tremors to optimize surgical maneuvering.
Challenges
The addition of robotic technology in mitral valve repair comes with significant financial investments, and procedures are more expensive than those performed through sternotomy. Procedure times are longer as well (2,8). Robotic surgery requires intense training in virtual, dry lab, and wet lab simulation, as well as team training, including mock operations, before a program can be started. Scheduling procedures is more challenging as there is a significant demand for robotic technology in other surgical disciplines. Slots for these operations are sometimes hard to organize, at least in the multidisciplinary setting. The same is true for having a stable team, including a competent patient-side assistant available.
The resectional parts of the procedure are possible once the mitral valve is well exposed. Finding the right amount of tissue that should be resected is probably the most significant challenge, and we recommend measuring the leaflet length directly with a ruler to define the targeted residual leaflet length. In cases of abundant myxomatous tissue, resection is probably easier than in cases where leaflet tissue is thin, like fibroelastic deficiency. The latter, chordal replacement, may be the safer option to avoid restriction of the posterior leaflet.
Conclusions
Robotic technology enables precise mitral valve visualization and assessment due to the integration of a 3D/HD camera. The surgeon is virtually immersed directly to the front of the valve. The multi-wristed robotic end effectors allow movements beyond the capabilities of standard thoracoscopic instrumentation. Therefore, leaflet resection maneuvers and repair of the mitral valve are enhanced and can be performed with a high comfort level. Robotic technology is an important adjunct to the armamentarium of minimally invasive mitral valve repair.
Acknowledgments
We thank the multidisciplinary teams at UPMC Presbyterian and UPMC Passavant Hospitals for all their contributions in establishing the robotic cardiac surgery program.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Robotic Mitral Valve Repair”. The article has undergone external peer review.
Peer Review File: Available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-45/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-45/coif). The series “Robotic Mitral Valve Repair” was commissioned by the editorial office without any funding or sponsorship. JB serves as an unpaid Guest Editor of the series. IS serves as an unpaid editorial board member of Journal of Visualized Surgery from August 2019 to July 2023. SFA was supported by the National Institutes of Health (No. F32HL165847), and a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund held by the University of Pittsburgh.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images and videos.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carpentier A, Loulmet D, Aupècle B, et al. Computer assisted open heart surgery. First case operated on with success. C R Acad Sci III 1998;321:437-42. [Crossref] [PubMed]
- Bonatti J, Kiaii B, Alhan C, et al. The role of robotic technology in minimally invasive surgery for mitral valve disease. Expert Rev Med Devices 2021;18:955-70. [Crossref] [PubMed]
- Fujita T, Kakuta T, Kawamoto N, et al. Benefits of robotically-assisted surgery for complex mitral valve repair. Interact Cardiovasc Thorac Surg 2021;32:417-25. [Crossref] [PubMed]
- Falk V, Seeburger J, Czesla M, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg 2008;136:1205-discussion 1205-6. [Crossref] [PubMed]
- Chan V, Mazer CD, Ali FM, et al. Randomized, Controlled Trial Comparing Mitral Valve Repair With Leaflet Resection Versus Leaflet Preservation on Functional Mitral Stenosis: The CAMRA CardioLink-2 Study. Circulation 2020;142:1342-50. [Crossref] [PubMed]
- Musumeci F, Ranocchi F, Lio A. The periareolar approach to robotic mitral valve repair. Ann Cardiothorac Surg 2022;11:548-9. [Crossref] [PubMed]
- Kiliç L, Şenay Ş, Ümit Güllü A, et al. Leyla loop: a time-saving suture technique for robotic atrial closure. Interact Cardiovasc Thorac Surg 2013;17:579-80. [Crossref] [PubMed]
- Williams ML, Hwang B, Huang L, et al. Robotic versus conventional sternotomy mitral valve surgery: a systematic review and meta-analysis. Ann Cardiothorac Surg 2022;11:490-503. [Crossref] [PubMed]
Cite this article as: Ashraf SF, Hasan I, Seese L, Deitz R, Yousef S, Sultan I, West D, Yoon P, Kaczorowski D, Bonatti J. Robotically assisted mitral valve repair—resectional techniques. J Vis Surg 2023;9:33.