Robotic thymectomy
Introduction
The first robotic thymectomy was reported in 2003 by Ashton et al. Since then, robotic assistance has been used with increased frequency in the performance of thymectomy. The anterior midline position of the thymus offers unique difficulties to even practitioners that are very experienced with video-assisted thoracoscopic surgery (VATS). Similar to VATS thymectomy, robotic thymectomy can be done with great safety and excellent intraoperative and perioperative results.
Indications
Robotic thymectomy can be performed for patients with normal thymuses (for instance in patients with myasthenia gravis without thymomas) and both benign and malignant tumors of the thymus. Invasion of the great vessels of the heart is generally a contraindication for minimally invasive thymectomy, whether VATS or robotic. Even large tumors of the thymus can be safely approached with robotic assistance.
Equipment
The Da Vinci Surgical System is currently the only FDA-approved robotic system for lung surgery. The surgeon sits at a console some distance from the patient who is positioned on an operating table in close proximity to the robotic unit with its four robotic arms. The robotic arms incorporate remote center technology, in which a fixed point in space is defined, and about it the surgical arms move so as to minimize stress on the thoracic wall during manipulations. The small proprietary Endowrist instruments attached to the arms are capable of a wide range of high-precision movements. These are controlled by the surgeon’s hand movements, via ‘master’ instruments at the console. The ‘master’ instruments sense the surgeon’s hand movements and translate them electronically into scaled-down micro-movements to manipulate the small surgical instruments. Hand tremor is filtered out by a 6-Hz motion filter. The surgeon observes the operating field through console binoculars. The image comes from a manoeuvrable high-definition stereoscopic camera (endoscope) attached to one of the robot arms. The console also has foot pedals that allow the surgeon to engage and disengage different instrument arms, reposition the console ‘master’ controls without the instruments themselves moving, and activate electric cautery. A second optional console allows tandem surgery and training. Da Vinci currently offers both the Xi and Si systems. The Xi system is newer and features an overhead beam that permits rotation of the instrument arms, allowing for greater flexibility in terms of direction of approach of the robot to the patient. Compared to the Si, he Xi also has thinner instrument arms, longer instruments themselves, and the option to switch the camera to any arm/port.
Preoperative evaluation
Preoperative evaluation of the patient undergoing thymectomy depends on the disease process in question. A thorough history and physical should be performed, with specific questions directed towards eliciting a history of myasthenia gravis (ptosis, diplopia, fatigable chewing dysarthria, dysphagia, fatigability). If myasthenia gravis is suspected then the appropriate serologic and electrophysiologic evaluation and treatment should be undertaken prior to operation to avoid perioperative complications. Patients with a myasthenia crisis should not undergo urgent or emergent thymectomy, but rather be treated medically with anticholinesterase inhibitors, intravenous immunoglobulin, and/or plasmapheresis until the disease is stabilized, and scheduled for elective thymectomy if they are otherwise appropriate candidates for the operation. Patients can generally be imaged with CT scan of the chest alone. Intravenous contrast is useful for assessing involvement of the major vessels, namely the innominate vein and superior vena cava. If a fat plane exists between the mass and the vessels then most likely robotic thymectomy can be attempted. If there is no fat plane or if the vessels are clearly involved then resection should generally be done via median sternotomy, although robotic exploration can be considered. If the lung appears to be involved, wedge resection can also be performed robotically. Although we generally perform robotic thymectomy via the right chest for reasons described below, a left sided location of the thymic mass may cause the surgeon to perform the operation via the left chest. Stress testing and/or pulmonary function testing is performed as indicated based on risk assessment via history and physical.
Patient positioning/port placement
Our typical approach is to perform thymectomy via the right chest, which we will describe here. A left-sided double lumen endotracheal tube is placed. We have found it simplest to place the tip of the double lumen tube in the trachea and then drive the pediatric bronchoscope into the left main stem bronchus via the left-sided lumen. The bronchoscope can then be used as a guide to cannulate the left main stem bronchus with the endotracheal tube. Positioning of the tube is then checked from the tracheal lumen with the pediatric bronchoscope. A small sliver of the cuff for the left side should be visible, and the right upper lobe bronchus should be clearly visualized in order to confirm correct placement of the tube. The endotracheal tube should be taped so that it is out of the way of the robot. Epidurals, Foley catheters, and arterial lines are generally unnecessary.
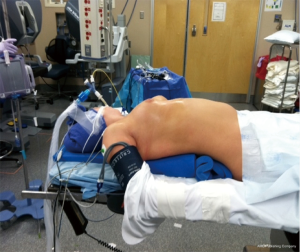
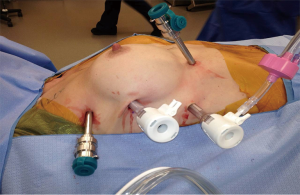
The patient is positioned supine with their body moved to the right edge of the bed, and the right side is elevated slightly with folded blankets or a gel pad and the right arm is allowed to hang down and secured on an arm board to expose the axilla (Figure 1). The patient’s chest is prepped and draped, making sure to expose the left chest (in case left VATS is needed), the entire sternum (in case emergent sternotomy is necessary to control bleeding), and the right chest all the way down to the bed. Port placement is depicted in Figure 2. The left robotic arm is placed high in the axilla. It is helpful to place an extra-long robotic port here to avoid collisions between the robotic arm and the patient’s shoulder. The camera port is placed halfway between the sternal notch and the xiphoid process. The right robotic arm port is placed close to the diaphragm and slightly more medial than the other ports, though not too close to the inferior horn of the thymus. A fourth robotic arm is generally not needed for the operation. The robotic arm ports for the DaVinci Xi system are 8 mm in diameter. For the Si system, the camera port is a 12-mm port and the arm ports are 8 mm. There should be at least 8–9 cm between each of the robotic ports. The assistant 12 mm port is placed so that it is triangulated behind the camera and right robotic arm ports. Carbon dioxide insufflation at 12 cm H2O is helpful, and can be delivered either via the assistant ports, or in the case of the Xi, the robotic arm ports. We avoid using insufflation on the robotic camera port because it tends to cause misting of the camera lens and the tubing can become kinked inadvertently as the camera is moved. The robot is then docked to the ports. With both the Xi and Si systems, the robot should approach the patient from the left side of the bed.
Conduct of operation
Any abnormalities of the chest wall, pleura, and lung are first noted and then biopsied or addressed, if indicated. Adhesions from prior surgery can be addressed with the robotic bipolar cautery; occasionally, the adhesions will need to be lysed thoracoscopically prior to placement of all the robot ports. The 8 mm robotic ports in the Si can be sized down with the rubber introducer cap to avoid losing the capnothorax when inserting 5 mm VATS instruments. The 8 mm robotic ports in the Xi have the 5 mm compatible valve built in.
A cadiere grasper is used in the left hand, and a thoracic bipolar dissector is used in the right.
Dissection of the thymus is started near the diaphragm, and carried anteriorly near the sternal edge, taking care to avoid the right internal mammary vessels. The surgeon can work their way up to junction of the mammary and the innominate vein, freeing the thymus from it. The thymus can then be dissected laterally (or towards the bottom of the screen) off the pericardium, avoiding the phrenic nerve.
Next, the right superior horn of the thymus is dissected off of the innominate vein, taking care to cauterize the small veins entering the thymus. Bleeding from these vessels can be generally controlled with pressure applied via a rolled up sponge, which should always be ready during the case in case a larger injury to the innominate vein occurs. The superior horn needs to be followed towards the thoracic inlet and brought down into the chest. The surgeon should take care to avoid undue trauma to the superior vena cava with their left robotic arm during this part of the operation.
Once the right superior horn is freed, dissection of the left side of the thymus inferiorly (toward the diaphragm) is performed. The left pleural space is opened. The thymus should be retracted into the right chest and towards the patient’s right shoulder. The left phrenic nerve can generally be seen from the right side if retraction is appropriate. Next, the left superior thymic horn is dissected off the innominate vein, which is below it. Again, the surgeon needs to be cognizant of not injuring the SVC and right internal mammary vein with the left robotic arm. The left internal mammary vein will enter the innominate vein near the apex of the left chest and should be avoided. The remainder of the dissection is then performed and the specimen removed with a Gore-Tex bag via the assistant port. A single flexible chest tube may be used to drain both the right and left pleural spaces. The gas insufflation is discontinued and each of the ports is removed to check for bleeding. The right lung is reinsufflated under direct vision via the camera and the camera port is finally removed. Figure 3 demonstrates our technique for robotic thymectomy.

Pearls/pitfalls
- Retraction is critical for bringing down the superior horns. Thorough extraction of the horns is essential for complete thymectomy. It is most helpful to alternate dissection above and below the superior horns to free it from the upper mediastinum and innominate vein;
- Injury to the innominate vein is the most feared complication of the surgery. A small injury may be amenable to topical hemostatic and pressure applied. A larger injury should be handled by having the area packed if possible, or if not possible, having the assistant applies pressure with a rolled up sponge. The robot can then be undocked and sternotomy can be performed;
- Interaction of the left robotic arm with the patient’s right shoulder is the most common technical issue. The shoulder should be positioned as described, as low as possible. Placing the port more anteriorly to give more clearance from the shoulder when the arm is directed towards the sternum and using a long robotic trocar are also helpful;
- Placement of the camera in the patient’s left side to visualize the left phrenic nerve is typically not necessary. A 30-degree down camera can be exchanged for the 0-degree camera and used if the nerve is not readily visible. If a tumor obscures the nerve, using a separate camera and left sided VATS port to help see the nerve can also be done. Some investigators have reported using the intravenous injection of indocyanine green and near-infrared fluorescent imaging to identify the phrenic nerve (2).
Results
Excellent perioperative and long-term outcomes are achievable with robotic thymectomy. Few complications occur, with a typical postoperative stay of 1–2 days and return to work or pre-thymectomy activities of daily living within around 2 weeks (3). Robotic thymectomy has been shown to lead to less blood loss, lower drain output, fewer chest tube days and a shorter hospital length of stay compared to sternotomy (4). Pain, quality of life, and return of function parameters are also improved for robotic thymectomy compared to sternotomy (5). Direct comparisons between robotic and VATS thymectomy have been few in number. Rückert et al. compared 79 VATS thymectomies with 74 robotic thymectomies and demonstrated similar operative time, similar conversion rates to sternotomy (1.2% robotic vs. 1.3% VATS), and similar perioperative morbidity (6). The investigators found an increased rate of complete remission of myasthenia gravis in the robotic group (39.2% vs. 20.3%, P=0.01). Others have found decreased pleural drainage duration and length of stay after robotic thymectomy compared to VATS thymectomy (1.1 vs. 3.6 days and 3.7 vs. 6.7 days respectively, P<0.01 for both) (7). Both of these studies are notable for potentially having surgeon experience and era effect confound these results. One consistent disadvantage of robotic thymectomy, though, has been increased cost mostly related to the robotic instruments (5,8).
Neurological outcomes for patients undergoing robotic thymectomy for myasthenia gravis has shown a 5-year rate of complete remission and overall improvement of 28.5% and 87.5% respectively, which is comparable to open and VATS series (9). Long-term results after thymectomy for early-stage thymoma have also been excellent, with a 5-year survival rate of 90%, though longer-term data with regards to survival and recurrence (a relevant consideration in this fairly indolent disease) remains unknown (10). Robotic thymectomy in pediatric patients and in patients with larger tumors and tumors involving lung, pericardium, and phrenic nerve have also been performed safely (11,12). We view invasion into the mediastinal vessels as the only indication for routine conversion (or initial approach via sternotomy, if obvious). We believe that the nature of the high-definitive 3-dimensional view of the robotic camera, increased dexterity, and easier ability to access the anterior mediastinum offer potential subjective benefits to the surgeon performing minimally invasive thymectomy over VATS.
Conclusions
Robotic thymectomy can be done safely and with minimal perioperative morbidity, and has become increasing common worldwide.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wei B, Cerfolio R. Robotic thymectomy. Asvide 2016;3:326. Available online: http://www.asvide.com/articles/1083
- Wagner OJ, Louie BE, Vallières E, et al. Near-infrared fluorescence imaging can help identify the contralateral phrenic nerve during robotic thymectomy. Ann Thorac Surg 2012;94:622-5. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Van Woerkom JM, et al. Long-term follow-up after robotic thymectomy for nonthymomatous myasthenia gravis. Ann Thorac Surg 2011;92:1018-22; discussion 1022-3. [Crossref] [PubMed]
- Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68-73; discussion e73.
- Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [Crossref] [PubMed]
- Augustin F, Schmid T, Sieb M, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery thymectomy. Ann Thorac Surg 2008;85:S768-71. [Crossref] [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [Crossref] [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [Crossref] [PubMed]
- Hartwich J, Tyagi S, Margaron F, et al. Robot-assisted thoracoscopic thymectomy for treating myasthenia gravis in children. J Laparoendosc Adv Surg Tech A 2012;22:925-9. [Crossref] [PubMed]
- Keijzers M, Dingemans AM, Blaauwgeers H, et al. 8 years' experience with robotic thymectomy for thymomas. Surg Endosc 2014;28:1202-8. [Crossref] [PubMed]
Cite this article as: Wei B, Cerfolio R. Robotic thymectomy. J Vis Surg 2016;2:136.