Staple-free robotic distal pancreatectomy and splenectomy
Introduction
Minimally invasive surgery has slowly gained popularity in the field of hepatopancreatobiliary surgery in the last few years. This is likely due to shorter length of stay, less estimated blood loss and postoperative pain, quicker recovery, and better cosmetic results (1-7). Laparoscopic distal pancreatectomy has become the standard of care for selected patients due to the absence of anastomosis and lower morbidity comparing to open surgery (4,8-10). The technical challenges of laparoscopic distal pancreatectomy are mainly due to the retroperitoneal location of the pancreas and its proximity to major vascular structures, and the high incidence of postoperative pancreatic fistula (3,5,11-13). Robotic technology adds several advantages to the traditional laparoscopic approach, such as a three-dimensional operative view with an enhanced hand-eye coordination, reduction of natural tremors, introduction of EndoWrist® technology, and a more comfortable and ergonomic position for the surgeon to operate (4,14). Here, we describe the operative technique of an entirely robotic distal pancreatectomy (RDP) with splenectomy. The method is presented in a stepwise approach along with a concise video.
Patient selection and workup
The distal pancreatectomy procedure is indicated for patients with lesions in the pancreatic body and tail. Several factors have to be taken in to account when a minimally invasive approach is considered. These include the patient’s body mass index (BMI), history of previous intra-abdominal procedures, cardio-pulmonary comorbidities and tolerance to general anesthesia. Disease specific factors include: histology (malignant vs. benign), size (bulky vs. small), and involvement of major vessels such as the celiac artery, common hepatic artery, superior mesenteric artery (SMA), and/or portal vein (PV). Involvement of the splenic vessels is not a contraindication in general.
Preoperative workup should include a three-phase pancreatic protocol CT scan or MRI. EUS guided fine needle biopsy is often indicated to determine the histology if neoadjuvant therapy is considered as part of the treatment plan.
Preoperative preparation
Routine preoperative evaluation is required, which includes routine lab work evaluating liver and kidney function, coagulation, etc. A mechanical bowel preparation is helpful especially when the patient has a history of chronic constipation.
Equipment preference card
The robotic platform we currently use is the DaVinci Xi (Intuitive Surgical, Sunnyvale, CA, USA). The following instruments are on the tray: EndoWrist Monopolar Hook Cautery, Scissors, Maryland Bipolar Forceps, Fenestrated Bipolar Forceps, Vessel Sealer, Medium-Large Clip Applier, Large Needle Driver and Large SutureCut™ Needle Driver, Tip-Up Fenestrated Grasper ×2.
Clinical summary
A 54-year-old male presented with an incidental pancreatic mass on CT scan. Further workup revealed an 8 cm × 6 cm well-differentiated neuroendocrine tumor involving the body of the pancreas. The tumor partially encased the splenic artery and vein but both vessels remained patent. The mass also abutted the PV and superior mesenteric vein (SMV) confluence. No evidence of distant metastasis.
Procedure (Figure 1)

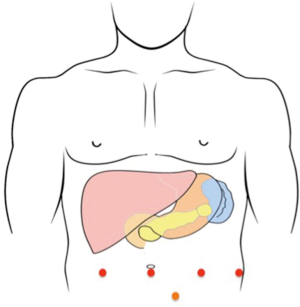
After induction of general anesthesia, the patient is placed in a split leg position. The skin is prepared and draped in a sterile fashion. An 8 mm infra-umbilical vertical incision is made and a pneumoperitoneum is established using a Veress needle. An 8 mm robotic trocar is placed and the robotic camera is introduced for exploration. Signs of carcinomatosis and/or liver metastasis are examined if intraabdominal adhesion is minimal. If nothing prohibitive is evidenced, we then place three additional 8 mm ports: one in the right upper quadrant and two in the left upper quadrant. All trocars are placed under direct visual guidance. A 5 mm bladeless trocar might be needed for the assistance. This is placed in the left lower quadrant (Figure 2).
All robotic trocars (red dots) are placed in a line. The robotic camera is placed in the infra-umbilical trocar. The assisting trocar (orange dot) is placed in the left lower quadrant.
After docking the DaVinci Xi system from the patient’s left side, the assistant is sitting between the legs to help with instrument exchange. We use the robotic vessel sealer to divide the ligamentum teres. This is helpful for instrument exchange, especially for obese patients. We then divide the greater omentum from the great curvature of the stomach. This is carried out from the pylorus up to the gastroesophageal junction. The arcade of gastroepiploic vessels is preserved. All short gastric vessels are divided with the vessel sealer if splenectomy is also performed. The stomach is retracted cephalad allowing entry to the lesser sac. This retraction can be facilitated by an 0 nylon suture around the body of the stomach. This nylon suture is brought out below the xiphoid using a Carter-Thompson needle. This will help suspend the left liver and stomach against the abdominal wall. A robotic hook cautery is used to dissect along the common hepatic artery and splenic artery; we then trace them back to the celiac axis and confirm the origin of the splenic artery. Lymphatic tissue along the artery can be dissected out as a separate specimen. The splenic artery is encircled with a vessel loop and divided with scissors after placing Hem-Lock clips. Then, careful dissection along the superior edge of the pancreas allows exposure of the gastroduodenal artery (GDA) and its confluence to the common hepatic artery. With the artery retracted up, the PV above the pancreas can be identified. We then dissect along the inferior edge of the pancreas to expose the SMV underneath the neck of the pancreas. A retropancreatic tunnel is carefully created bluntly between the neck of the pancreas and the PV. An umbilical tape is often used to wrap around the pancreatic neck and to lift the gland while we use the vessel sealer to divide the neck of the pancreas. Dissection is then carried out along the inferior edge of the pancreas laterally. The splenic flexure of the colon is carefully dissected off the inferior pole of the spleen. Then, with the divided end of the pancreas retracted to the left side, the dissection of the splenic vein is carried out. The splenic vein is encircled with a vessel loop and divided with scissors after placing Hem-Lock clips. We often use a 2–0 silk tie to ligate the splenic vein before we place the Hem-Lock clip. This is helpful when the tumor is bulky and the space is limited for clip placement. The tumor and pancreas are then fully mobilized from the retroperitoneum using the vessel sealer, from medial to lateral direction. The spleen is completely freed from the retroperitoneum. The specimen is placed in a large Endo-Catch bag. After confirming hemostasis, the stump of the pancreas can be closed with a 4–0 V-Lock suture in a running fashion. If a pancreatic duct can be identified, it should be suture closed with a 5–0 PDS suture. A Jackson-Pratt drain is placed near the site of pancreatic stump. Then we undock the DaVinci system from the trocars. The Endo-Catch bag with the specimen is then retrieved through a 7 cm long Pfannenstiel incision.
Postoperative management
On postoperative day (POD) 1 the nasogastric tube (NGT) was removed and diet advanced to a limited clear liquid diet (less than 60 cc/h). On POD 2 unlimited clear liquid diet was allowed. On POD 3 he was advance to a full liquid diet and the Foley catheter was removed. On POD 4 he was given a regular diet. On POD 6 his JP drain was removed and the patient was discharged home.
Tips, tricks and pitfalls
- All robotic trocars are placed in a line. This is necessary for the Xi system;
- Docking the DaVinci Xi system from the patient’s left side. The assistant sits between the patient’s legs to help with instrument exchange;
- The stomach is retracted cephalad by a 0 nylon suture around the body of the stomach. This nylon suture is brought out below the xiphoid using a Carter-Thompson needle. This will help suspend the left liver and stomach against the abdominal wall;
- The splenic artery has to be traced back to the celiac axis to confirm that the hepatic artery is not mistaken;
- An umbilical tape is often used to wrap around the pancreatic neck and to lift the gland while we use the vessel sealer to divide the neck of the pancreas;
- 2–0 silk tie is often used to ligate the splenic vein before we place the Hem-Lock clip. This is helpful when the tumor is bulky and the space is limited for clip placement;
- The stump of the pancreas can be closed with a 4-0 V-Lock suture in a running fashion. If a pancreatic duct can be identified, it should be suture closed with a 5-0 PDS;
- Skin needs to be prepared and draped wide enough for the Pfannenstiel incision.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ntourakis D, Marescaux J, Pessaux P. Robotic spleen preserving distal pancreatectomy: how I do it (with video). World J Surg 2015;39:292-6. [Crossref] [PubMed]
- Ryan CE, Ross SB, Sukharamwala PB, et al. Distal pancreatectomy and splenectomy: a robotic or LESS approach. JSLS 2015;19:e2014.00246.
- Adam MA, Choudhury K, Goffredo P, et al. Minimally Invasive Distal Pancreatectomy for Cancer: Short-Term Oncologic Outcomes in 1,733 Patients. World J Surg 2015;39:2564-72. [Crossref] [PubMed]
- Damoli I, Butturini G, Ramera M, et al. Minimally invasive pancreatic surgery - a review. Wideochir Inne Tech Maloinwazyjne 2015;10:141-9. [Crossref] [PubMed]
- Suman P, Rutledge J, Yiengpruksawan A. Robotic distal pancreatectomy. JSLS 2013;17:627-35. [Crossref] [PubMed]
- Parisi A, Coratti F, Cirocchi R, et al. Robotic distal pancreatectomy with or without preservation of spleen: a technical note. World J Surg Oncol 2014;12:295. [Crossref] [PubMed]
- Sharpe SM, Talamonti MS, Wang E, et al. The laparoscopic approach to distal pancreatectomy for ductal adenocarcinoma results in shorter lengths of stay without compromising oncologic outcomes. Am J Surg 2015;209:557-63. [Crossref] [PubMed]
- Sun Z, Zhu Y, Zhang N. The detail of the en bloc technique and prognosis of spleen-preserving laparoscopic distal pancreatectomy for pancreatic cancer. World J Surg Oncol 2015;13:322. [Crossref] [PubMed]
- Hori T, Masui T, Kaido T, et al. Laparoscopic Distal Pancreatectomy with or without Preservation of the Spleen for Solid Pseudopapillary Neoplasm. Case Rep Surg 2015;2015:487639.
- Postlewait LM, Kooby DA. Laparoscopic distal pancreatectomy for adenocarcinoma: safe and reasonable? J Gastrointest Oncol 2015;6:406-17. [PubMed]
- Sulpice L, Farges O, Goutte N, et al. Laparoscopic Distal Pancreatectomy for Pancreatic Ductal Adenocarcinoma: Time for a Randomized Controlled Trial? Results of an All-inclusive National Observational Study. Ann Surg 2015;262:868-73; discussion 873-4. [Crossref] [PubMed]
- Zhan Q, Deng X, Weng Y, et al. Outcomes of robotic surgery for pancreatic ductal adenocarcinoma. Chin J Cancer Res 2015;27:604-10. [PubMed]
- Sperlongano P, Esposito E, Esposito A, et al. Laparoscopic pancreatectomy: Did the indications change? A review from literature. Int J Surg 2015;21 Suppl 1:S22-5. [Crossref] [PubMed]
- Lai EC, Tang CN. Robotic distal pancreatectomy versus conventional laparoscopic distal pancreatectomy: a comparative study for short-term outcomes. Front Med 2015;9:356-60. [Crossref] [PubMed]
- Galvez D, Javed A, He J. Staple-free robotic distal pancreatectomy (RDP) and splenectomy. Asvide 2016;3:327. Available online: http://www.asvide.com/articles/1084
Cite this article as: Galvez D, Javed A, He J. Staple-free robotic distal pancreatectomy and splenectomy. J Vis Surg 2016;2:137.