Multidisciplinary treatment of benign tracheal stenosis—a case report
Highlight box
Key findings
• Surgery is the first line of treatment for laryngotracheal stenosis and leads to a high rate of success. Endoscopic treatment plays an important role for not yet stabilized subglottic stenosis and the management of restenosis.
What is known and what is new?
• The presence of the tracheotomy, diabetes mellitus and steroid therapy were negative prognostic factors for surgical intervention. In this case, the surgical resection/anastomosis could not achieve definitive results, but the endoscopic approach played an important role in complications management.
What is the implication, and what should change now?
• Multidisciplinary approach is the key to managing tracheal stenosis and complications from resection/anastomosis surgery.
Introduction
Background
Benign stenosis involving the subglottic region represents a major therapeutic challenge (1). Surgery is the first line of treatment for tracheal stenosis and leads to a high rate of success (2,3). In recent years, the interest for endoscopic treatment modalities has increased (4-8), with the aim to treat patients with benign subglottic stenosis not yet stabilized, avoiding the need of a tracheostomy, which would complicate surgical repair. Laser-assisted endoscopy, with or without stenting, has however rarely been used in subglottic stenosis for anatomical and technical reasons (9). Additionally, endoscopic treatment plays a role also in management of re-stenosis after surgical resection.
Rationale and knowledge gap
Herewith, we report a case of a 60-year-old male patient that came to our attention with a tracheotomy and subglottic stenosis, related with a previous recovery in an intensive care unit for a myasthenic crisis, that required prolonged intubation and mechanical ventilation. The complexity of the case was represented not only by the length of the stenosis and the presence of tracheotomy, but also by diabetes mellitus, myasthenia gravis and steroid therapy. We were fully aware of the presence of well-documented risk factors of resection/anastomosis, as reported by Wright et al. (10) and Auchincloss et al. (11), thus we explained to the patient all possible therapies and their complications in a multidisciplinary setting.
Objective
Considering the young age of the patient, we proposed surgical intervention. The close collaboration between thoracic surgeons and thoracic endoscopists permitted to manage the complications of such intervention.
All photographs are completely unidentified and there are no details on the patient mentioned within the text. We present this case in accordance with the CARE reporting checklist (available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-24/rc).
Case description
A 60-year-old male affected by systemic arterial hypertension and non-insulin-dependent diabetes mellitus came to our attention with a subglottic stenosis and a tracheostomy.
Sixteen months earlier the patient presented with diplopia and asthenia onset, thus myasthenia gravis was diagnosed, thanks to neurologic exams and antibodies dosage (anti-acetylcholinesterase antibodies positivity). The patient was treated with immunoglobulin and anticholinesterase therapy, but two months later a myasthenic crisis occurred. The patient was intubated and an intensive care unit recovery with mechanical ventilation was required for 16 days. After extubating, the patient was sent to rehabilitation for a period of one month, achieving good results: he got back to his previous routine life.
Almost 1 year later the patient presented with an episode of dyspnea associated with tirage and cornage. Therefore, a bronchoscopic control was performed, showing subtotal tracheal stenosis, and an emergency tracheotomy was performed.
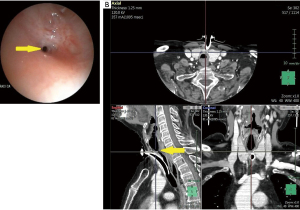
When the patient arrived at our attention bronchoscopy showed a sub-total tracheal stenosis above tracheal cannula, with a residual tracheal lumen of 1–2 mm, at a distance of 2.5 cm from vocal cords (Figure 1A, Video 1). Neck and chest computed tomography (CT) scans showed a distance between the stenosis and inferior limit of the tracheotomy of about 4 cm and the involvement of the tracheal wall (Figure 1B).
After neurological evaluation, myasthenia gravis resulted well controlled with steroid therapy (deltacortene 37.5 mg/day), without anticholinesterase drugs. Therefore, after a multidisciplinary discussion, involving thoracic surgeon, thoracic endoscopist and neurologist, considering the young age, surgical intervention (resection/anastomosis) was proposed to the patient and all possible complications (related with steroid therapy and length of stenosis), and other possible therapies were informed. One month later, the patient was admitted to our Unit for preoperative analysis, including another bronchoscopy, with bronchial washing, finding no pathogens but normal flora, and a smear that resulted negative. Thus, after informed consent obtainment, the patient underwent tracheal resection and reconstruction under general anesthesia. Ventilation was initially accomplished through tracheotomy, but an orotracheal tube was positioned above the stenosis.
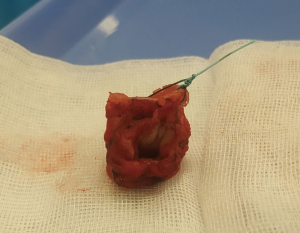
Through a collar incision, the inferior margin of the stenosis was identified and resected, thus ventilation was guaranteed with an armored tube (number 6, flexometallic tube, Teleflex Medical S.r.l.) placed into the distal trachea. The upper margin of the stenosis was identified intraoperatively with bronchoscopy, as reported in the literature (1). The total length of resection was 4 cm (Figure 2). The anastomosis was performed with a running suture (3-0 absorbable monofilament) for pars membranacea and interrupted sutures (2-0 absorbable monofilament) tied on the outside for pars cartilaginea. Before completing the anastomosis, the tube was removed from the field and the orotracheal tube was progressed into the distal trachea. The anastomotic line was supported by the thyroid isthmus and strap muscle. At the end, a stitch (non-absorbable suture) was placed from the chin skin to presternal skin, in order to avoid neck hyperextension the patient was extubated and the postoperative bronchoscopic control showed a regular anastomosis.
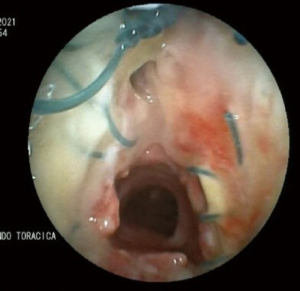
The patient was closely monitored for three days in our sub-intensive care unit. Cervical drainage was removed on the 3rd postoperative day. On the 5th postoperative day patient presented with wound infection, treated with daily medications (partial opening of skin suture and daily iodine gauze packing) however on the 10th postoperative day cervicotomy air leak occurred and compressive wound medication was performed Bronchoscopy revealed a dehiscence of left anterolateral wall of the trachea (Figure 3) and neck and chest CT scan showed the presence of cervical abscess.
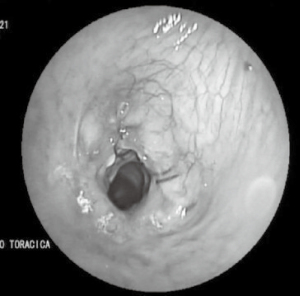
Therefore, the patient underwent surgical revision with gauze packing and a cervical drainage was left in place. A targeted antibiotic therapy with Piperacillin/Tazobactam (4.5 g three times a day for 14 days for intravenous infusion) and Vancomycin (intravenous loading dose of 1,750 mg for the first day and then 1,250 mg twice a day) was set, after identification of Corynebacterium striatum and Staphylococcus aureus on swab, and empirical therapy with ceftriaxone 2 g/day was interrupted. Bronchoscopic controls performed during the hospital stay showed progressive closure of the dehiscence and a concomitant progressive fibrotic evolution of the anastomosis with tracheal lumen reduction (about 6 mm) (Figure 4).
Thus, on the 62nd postoperative day, a balloon dilatation up to 11 mm (CRETM Single-Use Pulmonary Balloon Dilatation Catheter, Boston Scientific) of the re-stenosis was performed at the level of the anastomosis, under general anesthesia with flexible bronchoscope. The patient was discharged 4 days later (66th postoperative day), in absence of respiratory symptoms.
However, after one month, patient presented dyspnea, tirage and cornage, and a bronchoscopic control revealed a re-stenosis of the anastomosis (residual caliber less than 50% of the tracheal lumen) (Video 2). Therefore, the patient underwent tracheal dilatation with rigid bronchoscope (Efer Medical, La Ciotat, Cedex, France) with progressive increasing caliber until black bronchoscope and placement of a silicone stent (NOVATECH® GSS™ TD) with a diameter of 16 mm and a length of 4 cm in order to stabilize the tracheal wall. The upper margin of the stent was at distance of about 5 mm from the vocal cords (Figure 5A).
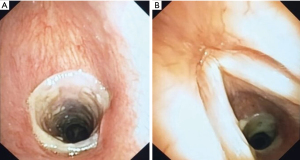
The patient underwent bronchoscopic control two months later without stent dislocation, in absence of granulomas or secretions obstruction (Figure 5B). Currently the patient is in good clinical condition, without symptoms. Next control is programmed in six months, in order to evaluate the possibility of stent removal.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The innovation in respiratory intensive care units allowed a prolonged management of patients needing mechanical ventilation, but it is estimated that about 2–3% of patients who undergo intubations and/or tracheotomy will develop tracheal stenosis (12), and usually, when symptoms occurred, the trachea is narrowed up to 75% of its lumen (13).
The endotracheal tube cuff can cause a pressure-induced ischemic injury of the tracheal wall, with subsequent circumferential scarring and narrowing of the involved trachea. On the other hand, percutaneous tracheotomy may cause a direct damage of trachea wall. In our case, the stenosis was firstly related with prolonged intubation and an emergency tracheotomy added the disadvantage of an increased length of tracheal resection.
Key findings
When the patient came to our attention, he presented a complex subglottic stenosis with a residual lumen of 2 mm, with a tracheotomy. We knew that the presence of the tracheotomy, diabetes mellitus and steroid therapy were negative prognostic factors for surgical intervention, thus we discuss it with the patient in a multidisciplinary setting, specifying all possible therapeutic approaches and their adverse events. The patient was really motivated for surgical intervention, which represents the treatment of choice, according to many authors that reported a high success rate of more than 90% (2,3,8). Unfortunately, postoperative course was complicated by wound infection with a partial dehiscence of the anterolateral part of the anastomosis.
Comparison with similar researches
This adverse event was reported also in several papers, where dehiscence ranged between 1% and 6% (2,14,15). Different factors probably contributed to this complication, such as the length of the resected trachea (10), the tracheotomy, which was often associated with airway colonization, as observed by Ciccone et al. (10,16,17). The partial dehiscence was managed with drainage, antibiotics and conservative therapy, not requiring surgical re-intervention or temporary positioning of a Montgomery T-tube (2). However, it resulted in a progressive re-stenosis. This complication was one of the most frequent anastomotic complications described in the literature, where its incidence varied between 1.5% and 13.4% (2,14,15).
In these cases, the endoscopic approach represented the only possible conservative way to manage the critical situation, aimed to avoid more invasive procedures such as re-tracheotomy, that probably would have been permanent. In our case, we firstly choose balloon dilatation with flexible bronchoscope, obtaining an 11-mm lumen diameter, due to the recent dehiscence of anastomosis, in order to avoid tracheal laceration, as reported by Kim et al. (18). Subsequent endoscopic controls showed stenosis recurrence. This event is frequently described in the literature after balloon dilatation for benign tracheal stenosis (6,19), and Lee et al. reported a recurrence rate of 80% (20), but we firstly preferred this option because of the recent closure of anastomotic dehiscence. Thus, we decided to treat the re-stenosis with progressive mechanical dilation with rigid bronchoscope and stent positioning in order to support the airway walls. Rigid bronchoscopy offered the possibility of a dilation under direct visual control, permitting the introduction of several tools at the same time (laser fiber for coagulation, dilators, rigid suction tubes) in order to better front complications. It supported the airways’ patency allowing ventilation and oxygenation, and, at least but not at last, rigid bronchoscope permitted stent placement. In the STROBE trial, the authors compared the clinical efficacy of balloon dilatation and stent positioning for treatment of tracheal stenosis with a follow-up of 2 years. This study showed that stent placement, with removal 1 year later, had a better effect on long-term stabilisation of tracheal patency in respect to balloon dilatation technique, also in patients with complex stenosis (19). The stent was a temporary item aimed at defending the airway calibre, till the fibro-cicatricial process end, as reported by several authors (5-8), and for this reason silicon stent was recommended because it could be easily removed, in respect to metallic or combined ones. Also in the study of D’Andrilli et al., restenosis after surgical intervention (8 cases) was treated with laser-assisted dilatation and silicon stent positioning (15).
A possible complication of stent positioning was dislocation, especially for subglottic stenosis, but at the moment, in our case it did not occur, even if the stent was really near vocal cords (5 mm), probably due to the characteristics of the fibrous stricture, which was stiff and rigid. In 2011, Foccoli et al. observed that laser-assisted mechanical dilation with or without stenting was efficacious in 66% of complex stenoses, with very few transient complications and no mortality (5). The bronchoscopic control, performed 2 months after stent positioning, showed the absence of granulomas, which represents one of the other most frequent complications (7,8), and no stent obstruction by sticky secretions or bacterial over infections. The patient was carefully informed about the necessity of daily aerosol therapy to avoid stent plugging with secretions. At the subsequent bronchoscopic control, the stent locked in place without granulomatous tissue nor secretions obstruction. Our aim is to maintain stent for 10–12 months, evaluating its removal after 1 year as reported in a study of Galluccio et al. (8) on 209 patients with tracheal stenosis endoscopically treated, that reported a mean duration of stent permanence of 10±6 months, for simple stenoses, and 20±3 months for complex ones.
Strengths and limitations
In our case, the multidisciplinary approach is the key to managing tracheal stenosis and complications from resection/anastomosis surgery. The surgical resection and anastomosis can not achieve definitive results, nonetheless the endoscopic approach plays an important role in preoperative case selection and in complications management. This paper has some limitations related to its “case-report” nature. Firstly, it described a single patient and did not permit to generalize. Secondly, it cannot allow to identify a cause-effect relationship. However, our aim was simply to describe the diagnostic-therapeutic process of this particular patient, sharing our clinical experience and drawing some possible food for thought.
Conclusions
Close collaboration between thoracic surgeons and thoracic endoscopists represents the key to the treatment and management of complex tracheal stenosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-24/rc
Peer Review File: Available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-24/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-24/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grillo HC. Primary reconstruction of airway after resection of subglottic laryngeal and upper tracheal stenosis. Ann Thorac Surg 1982;33:3-18. [Crossref] [PubMed]
- Rea F, Callegaro D, Loy M, et al. Benign tracheal and laryngotracheal stenosis: surgical treatment and results. Eur J Cardiothorac Surg 2002;22:352-6. [Crossref] [PubMed]
- Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-92; discussion 492-3. [Crossref] [PubMed]
- Giudice M, Piazza C, Foccoli P, et al. Idiopathic subglottic stenosis: management by endoscopic and open-neck surgery in a series of 30 patients. Eur Arch Otorhinolaryngol 2003;260:235-8. [Crossref] [PubMed]
- Foccoli P, Scappaticci E, Rea F, et al. Management of post-intubation and/or tracheotomy tracheal stenoses. Monaldi Arch Chest Dis 2011;75:82-5. [PubMed]
- Cavaliere S, Bezzi M, Toninelli C, et al. Management of post-intubation tracheal stenoses using the endoscopic approach. Monaldi Arch Chest Dis 2007;67:73-80. [PubMed]
- Mandour M, Remacle M, Van de Heyning P, et al. Chronic subglottic and tracheal stenosis: endoscopic management vs. surgical reconstruction. Eur Arch Otorhinolaryngol 2003;260:374-80. [Crossref] [PubMed]
- Galluccio G, Lucantoni G, Battistoni P, et al. Interventional endoscopy in the management of benign tracheal stenoses: definitive treatment at long-term follow-up. Eur J Cardiothorac Surg 2009;35:429-33; discussion 933-4. [Crossref] [PubMed]
- Shapshay SM, Beamis JF Jr, Hybels RL, et al. Endoscopic treatment of subglottic and tracheal stenosis by radial laser incision and dilation. Ann Otol Rhinol Laryngol 1987;96:661-4. [Crossref] [PubMed]
- Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg 2004;128:731-9. [Crossref] [PubMed]
- Auchincloss HG, Wright CD. Complications after tracheal resection and reconstruction: prevention and treatment. J Thorac Dis 2016;8:S160-7. [PubMed]
- Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am J Med 1981;70:65-76. [Crossref] [PubMed]
- Honings J, Gaissert HA, Ruangchira-Urai R, et al. Pathologic characteristics of resected squamous cell carcinoma of the trachea: prognostic factors based on an analysis of 59 cases. Virchows Arch 2009;455:423-9. [Crossref] [PubMed]
- Bibas BJ, Terra RM, Oliveira AL Junior, et al. Predictors for postoperative complications after tracheal resection. Ann Thorac Surg 2014;98:277-82. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Andreetti C, et al. Long-term results of laryngotracheal resection for benign stenosis from a series of 109 consecutive patients. Eur J Cardiothorac Surg 2016;50:105-9. [Crossref] [PubMed]
- Ciccone AM, De Giacomo T, Venuta F, et al. Operative and non-operative treatment of benign subglottic laryngotracheal stenosis. Eur J Cardiothorac Surg 2004;26:818-22. [Crossref] [PubMed]
- Piazza C, Del Bon F, Paderno A, et al. Complications after tracheal and cricotracheal resection and anastomosis for inflammatory and neoplastic stenoses. Ann Otol Rhinol Laryngol 2014;123:798-804. [Crossref] [PubMed]
- Kim JH, Shin JH, Shim TS, et al. Deep tracheal laceration after balloon dilation for benign tracheobronchial stenosis: case reports of two patients. Br J Radiol 2006;79:529-35. [Crossref] [PubMed]
- Marchioni A, Andrisani D, Tonelli R, et al. Stenting versus balloon dilatation in patients with tracheal benign stenosis: The STROBE trial. Laryngoscope Investig Otolaryngol 2022;7:395-403. [Crossref] [PubMed]
- Lee KH, Ko GY, Song HY, et al. Benign tracheobronchial stenoses: long-term clinical experience with balloon dilation. J Vasc Interv Radiol 2002;13:909-14. [Crossref] [PubMed]
Cite this article as: Fanucchi O, Picchi A, Marrama E, Ambrogi MC, Lucchi M, Ribechini A. Multidisciplinary treatment of benign tracheal stenosis—a case report. J Vis Surg 2023;9:37.