Minimally invasive central pancreatectomy and pancreatogastrostomy: current surgical technique and outcomes
Introduction
With improvements in surgical techniques and perioperative care, the mortality associated with pancreatic resection (PR) has decreased dramatically; however the morbidity associated with this procedure remains high. Centrally located lesions pose a particular challenge due to the variety of options available for surgical resection. Patients may undergo pancreaticoduodenectomy (PD), distal pancreatectomy (DP) or central pancreatectomy (CP) depending on the size, location and malignancy potential of the lesion (1). CP (also known as middle pancreatectomy or median pancreatectomy) was first ascribed to Ehrhardt in 1908 (2,3). Guillemin and Bessot performed the first CP with pancreato-enteric reconstruction in 1957 for chronic pancreatitis, and subsequently Dagradi and Serio described the operation for resection of a benign lesion (insulinoma) in 1982 (1,4-6). The primary aim of performing a CP is the preservation of both endocrine and exocrine function of the pancreas while still maintaining oncologic efficacy (7). Specifically, for centrally located low-grade lesions, a DP or PD obligates a substantial volume of the pancreas removed, placing patients at higher risk of post-operative diabetes and exocrine dysfunction (7-9). In comparison to DP, CP also allows for preservation of spleen (7). Advantages of CP over PD include reduced mortality and preservation of the duodenum and bile duct, with only a single anastomosis needed for reconstruction as opposed to multiple anastomoses required for restoring continuity of the hepatic duct, pancreatic duct and intestinal tract (1,10). The concerns surrounding CP include high incidence of post-operative pancreatic fistula (POPF) and potentially inadequate oncologic resection in cases of malignancy (9). However, studies have shown that although the rates of POPF after CP are relatively high (20–50%), oftentimes these patients possess soft glands and small ducts, which are both well-established risk factors for POPF (9). Also, in most cases the POPF is clinically insignificant [International Study Group on Pancreatic Fistula (ISGPF) grade A] (9). Therefore, CP is a reasonable approach for centrally located, benign or low-grade pancreatic lesions that allows preservation of pancreas parenchyma and adjacent organs.
With increasing utilization of laparoscopic and robotic pancreatectomy, patients can now undergo either an open or minimally invasive surgery (MIS) procedure. Laparoscopic and robotic PD has gained interest due to comparable morbidity, mortality and oncologic outcomes versus open PD when performed in select patients (11-15). For DP, MIS approach has now become the standard of care due to its favorable outcomes in comparison to open DP (11,16). In a recent meta-analysis comparing 1,814 patients undergoing open versus laparoscopic DP, the laparoscopic approach resulted in less blood loss, shorter hospital length of stay (LOS), fewer surgical site infections and lower morbidity (17). Similarly, a MIS approach for CP has become increasingly common with the goal of decreasing the impact of morbidity related to the decreased size of incisions, shorter hospital stays, and shorter time until return to work. While both laparoscopic and robotic CP are being performed, laparoscopy may be somewhat limited given the restricted workspace and the inability to articulate instruments in a manner requisite for these complex procedures (18-21). These limitations are potentially alleviated by the use of robotic surgery. Herein, we report our technique of performing an MIS CP, with accompanying video demonstration of the key portions of the operation. Indications for CP and a brief summary of outcomes following CP are also discussed.
Indications for CP
Pancreatic lesions of the central pancreas can be extirpated via numerous operative approaches depending on their size, location and pathology. Extended PD or near-total DP are performed for pancreatic ductal adenocarcinoma (PDAC) or main-duct-type intraductal papillary mucinous neoplasm (IPMN) with potential invasive component, in order to achieve adequate resection of the tumor and also the surrounding lymph nodes, which is not always achievable with CP (7,8). However, for low-grade malignant tumors or benign lesions, use of PD or DP would consequently remove much of the normal pancreatic parenchyma which is likely of no therapeutic benefit (Figure 1). Enucleation can also be considered; however, this should not be the procedure of choice for malignant tumors or benign lesions greater than 2 cm, or location adjacent to the main pancreatic duct (19). Therefore, CP may be an appropriate alternative for a subset of patients possessing low-grade malignant tumors or benign lesions restricted to the central pancreas (19). The most common indications for CP include neuroendocrine tumors followed by cysts that display indeterminate characteristics such as branch-duct-type IPMNs, and solid pseudopapillary neoplasms (Table 1) (1,7,8,19). Contraindications to this procedure include PDAC, main-duct-type IPMN, neoplastic involvement of adjacent organs, and large lesions where it is impossible to preserve the left pancreatic stump (2).
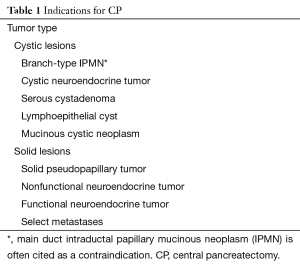
Full table
Surgical technique and technical aspects of MIS CP
All patients should be evaluated for a pancreatic lesion using a pancreas protocol CT or MRI and serum CA19-9 levels when deemed necessary. If a patient is found to have a lesion amenable to resection via an MIS CP, a preoperative assessment by an anesthesiologist is performed and medical clearance should occur similar to an open approach.
After surgical consent has been obtained, the patient is placed in a supine position with right arm extended to 90o and the left arm is tucked. Intravenous access, monitoring lines, and a Foley catheter are placed. A nasogastric tube is inserted for stomach decompression. The abdomen is prepped and draped in the standard manner. Safe entry to the abdomen is obtained via the Hassan technique (supraumbilical) or a Veress needle. The abdomen is then insufflated to 15 mmHg and a camera port is placed in the periumbilical position (12 mm). A 5 mm port for the liver retractor is placed in the right anterior axillary line. Subsequent ports include two right-sided abdominal robotic ports (8 mm) and a left-sided abdominal port (8 mm). The exact location of the robotic ports depends on whether a Si or Xi robot (da Vinci® Surgical System) will be used. The assistant port is placed in the left lower quadrant and should be 12 mm in order to accommodate a laparoscopic stapler. The robot is then docked over the patient’s head or towards their left in case of the SI or XI robot (da Vinci® Surgical System) respectively.
Although the indications for MIS CP are generally low-grade neoplasms or benign tumors, inspection of the abdominal cavity and surface of the liver is performed to identify any pathologic implants. Subsequently, the lesser sac is entered with the vessel-sealing device and the gastrocolic omentum is dissected free from the stomach while preserving the gastroepiploic vessels. This dissection is carried from the pylorus up along the greater curvature of the stomach to allow elevation of the stomach and adequate exposure of the anterior surface of the pancreas. At this stage, if the lesion cannot be readily visualized, ultrasound can be used to delineate the extent of the tumor and its relationship to the surrounding structures. The inferior border of the pancreas is mobilized and SMV is identified coursing posterior to the pancreatic neck. The superior border of the pancreas is also mobilized, and the common hepatic artery, gastroduodenal artery and portal vein are identified. Once both the inferior and superior borders of the pancreas have been mobilized, tunneling is performed behind the neck of the pancreas to dissect the pancreas free from the superior mesenteric vein/portal vein. Dissection is then performed in a medial-to-lateral manner to free the undersurface of the pancreas from the splenic vein. The splenic artery may follow a tortuous course behind the pancreas or through the pancreas, highlighting the necessity for a meticulous dissection to avoid injury to this vessel or the underlying splenic vein. While dissecting the central pancreas free from the splenic artery, caution must be taken to identify the overlying coronary vein (left gastric vein), which in our experience serves as a critical anatomic landmark of the celiac trunk. This vein can be ligated if necessary. The dissection of the central pancreas from the splenic vein and artery is continued until the distal extent of the tumor has been reached. Liberal use of intraoperative ultrasound can confirm the location of the tumor and a duplex can also confirm arterial/venous anatomy. The plane of transection of the pancreas to the left of the tumor is identified and marked to represent the distal margin of the specimen during pathological examination, and the transverse pancreatic arteries are suture ligated. The pancreatic neck to the right of the tumor is routinely divided with a GIA stapler. The parenchyma located to the left of the tumor is then transected with cautery scissors or a thermal device in order to allow for identification of the pancreatic duct, which will later be sewn to the intestinal mucosa. The specimen is placed in a 15 mm Endo CatchTM (Covidien, New Haven, CT) bag and removed through the accessory left lower quadrant port. The specimen is then sent to confirm pathological diagnosis and ensure adequate margins. At this point, if the pathology is confirmed as a benign tumor or a low-grade neoplasm, we proceed with the reconstruction. However, if the pathology is found to be malignancy or high-grade neoplasm, we believe a PD or DP should be performed.
Reconstruction following CP can be performed by either a pancreatogastrostomy or a Roux-en-y pancreaticojejunostomy. Pancreatogastrostomy is favored at our institution due to the formation of a single anastomosis (in comparison to roux-en-y pancreaticojejunostomy) and maintenance of physiologic drainage (7). The transected surface of the pancreatic head is oversewn using a running V-LocTM (Medtronic, Minneapolis, MN, USA) suture to ensure hemostasis. Attention is then paid to the reconstruction of the pancreatogastrostomy. The stomach is allowed to lie flat in the retroperitoneum and an optimal location in close proximity to the transected pancreas is marked with a marking pen. At this stage the pancreatic tail should be mobilized further to ensure enough mobility out of the retroperitoneum for a tension free anastomosis. Corner sutures are placed to anchor the pancreas to the stomach on the cranial and caudal aspect of the pancreas. The anterior surface of the pancreas is sutured to the posterior surface of the stomach to create the ‘back row’ of the pancreatogastrostomy, using a running V-LocTM suture. A small gastrotomy is created and duct-to-mucosa anastomosis is performed with simple interrupted 5–0 absorbable monofilament sutures over a 5-Fr pediatric feeding tube as a stent in the pancreatic duct. The posterior surface of the pancreas is then sutured to the stomach using a running V-LocTM suture, completing the outer layer of the anastomosis. All layers of the pancreatogastrostomy are performed using running V-LocTM sutures except the duct-to-mucosa layer, which we perform in an interrupted manner with 5–0 absorbable monofilament sutures. In the event that the non-dilated pancreatic duct is too small to visualize, we perform an invagination by making a larger gastrotomy and suturing the entire face of the gland into the stomach itself (similar manner to description above), utilizing two layers.
There are multiple members of the team that are critical for success of this operation. This includes anesthesiologists and anesthetists that monitor the airway and stability of the patient, the surgeon who is at the console following port-placement, and the surgical trainee or assistant who is at the bedside, and is responsible for assisting with port-placement, docking of the robot, instrument exchanges and providing help during the operation through the assistant port. Additionally, a scrub nurse is important for providing the appropriate instruments and suture as well as a circulator nurse who maneuvers the robot patient cart to the bedside and is able to acquire any instruments or suture that is not on the operative field. This multi-disciplinary approach ensures a cohesive and safe operation (Figure 2).

Outcomes of MIS CP
With a recent increase in the use of cross-sectional imaging, there has been a concomitant increase in the identification of low-grade and benign pancreatic lesions which are amenable to a CP (23). Therefore, an increasing number of patients are now undergoing CPs and have been reported. In select higher volume series on open CP, mean morbidity was found to be 50.3% and mortality 0.7% (1,7-10,24-33) (Table 2). The mean re-operative rate was 3.9%. Rates of POPF (34.1%) are comparable to those reported for PD and DP (8,34), while postoperative diabetes mellitus (DM) (3.2%), and exocrine insufficiency (EI) (6.5%) are a relatively infrequent complication. In comparison to open series, the quantity of patients reported in MIS series of CP is even more limited (20,21,35,36). The largest series on laparoscopic CP was performed by Rotellar and colleagues, which included nine patients (20). In this group of patients, morbidity was 44% including one reoperation (11%) and two patients who developed POPF (22%); there were no mortalities and no patients experienced endocrine or EI. The largest series of robotic CP was reported by Abood and colleagues, and also included outcomes for 9 patients with low-grade neoplasms (19). In this series, there was one conversion to an open procedure and 78% of patients experienced a POPF, with clinically significant pancreatic fistula occurring in 22%. This coincides with the rates published for open CP (median =21.2%), where most often only clinically significant fistulas were noted (1,7,9,10,25-27,37). There were no cases of EI or endocrine dysfunction, and Clavien grade III or higher complications occurred in one patient (11%) with no reoperations or mortality. Similar outcomes were seen in additional reports of robotic CP, indicating it is a viable approach to select central pancreas lesions in specialized centers (18,19,23,38,39) (Table 3).
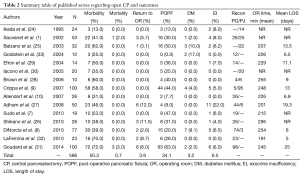
Full table
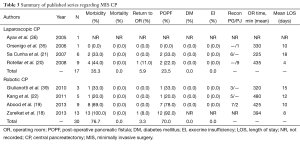
Full table
Conclusions
Robotic CP is safe and efficacious for lesions located in the central pancreas. This approach is likely to gain acceptance for select patients that have benign or low-grade neoplasms in the central pancreas given preservation of pancreatic volume and avoidance of adjacent organ resection. Furthermore, robotic CP can achieve similar outcomes with comparable rates of mortality and morbidity as the open approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sauvanet A, Partensky C, Sastre B, et al. Medial pancreatectomy: a multi-institutional retrospective study of 53 patients by the French Pancreas Club. Surgery 2002;132:836-43. [Crossref] [PubMed]
- Iacono C, Ruzzenente A, Bortolasi L, et al. Central pancreatectomy: the Dagradi Serio Iacono operation. Evolution of a surgical technique from the pioneers to the robotic approach. World J Gastroenterol 2014;20:15674-81. [Crossref] [PubMed]
- Finney JM. VII. Resection of the Pancreas: Report of a Case. Ann Surg 1910;51:818-29. [Crossref] [PubMed]
- Fagniez PL, Kracht M, Rotman N. Limited conservative pancreatectomy for benign tumours: a new technical approach. Br J Surg 1988;75:719. [Crossref] [PubMed]
- Guillemin P, Bessot M. Chronic calcifying pancreatitis in renal tuberculosis: pancreatojejunostomy using an original technic. Mem Acad Chir (Paris) 1957;83:869-71. [PubMed]
- Dagradi A, Serio G. Pancreatectomia intermedia. In: Enciclopedia medica italiana. Pancreas, vol. XI. Florence: USES Ed. Scientifiche, 1984:850-1.
- Sudo T, Murakami Y, Uemura K, et al. Middle pancreatectomy with pancreaticogastrostomy: a technique, operative outcomes, and long-term pancreatic function. J Surg Oncol 2010;101:61-5. [Crossref] [PubMed]
- DiNorcia J, Ahmed L, Lee MK, et al. Better preservation of endocrine function after central versus distal pancreatectomy for mid-gland lesions. Surgery 2010;148:1247-54; discussion 1254-6. [Crossref] [PubMed]
- Crippa S, Bassi C, Warshaw AL, et al. Middle pancreatectomy: indications, short- and long-term operative outcomes. Ann Surg 2007;246:69-76. [Crossref] [PubMed]
- Allendorf JD, Schrope BA, Lauerman MH, et al. Postoperative glycemic control after central pancreatectomy for mid-gland lesions. World J Surg 2007;31:164-8; discussion 169-70. [Crossref] [PubMed]
- Stauffer JA, Asbun HJ. Minimally invasive pancreatic surgery. Semin Oncol 2015;42:123-33. [Crossref] [PubMed]
- Buchs NC, Addeo P, Bianco FM, et al. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg 2011;35:2739-46. [Crossref] [PubMed]
- Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc 2012;26:2397-402. [Crossref] [PubMed]
- Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg 2012;215:810-9. [Crossref] [PubMed]
- Kendrick ML. Laparoscopic and robotic resection for pancreatic cancer. Cancer J 2012;18:571-6. [Crossref] [PubMed]
- Kooby DA, Hawkins WG, Schmidt CM, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg 2010;210:779-85, 786-7. [Crossref] [PubMed]
- Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59. [Crossref] [PubMed]
- Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013;258:554-9; discussion 559-62. [PubMed]
- Abood GJ, Can MF, Daouadi M, et al. Robotic-assisted minimally invasive central pancreatectomy: technique and outcomes. J Gastrointest Surg 2013;17:1002-8. [Crossref] [PubMed]
- Rotellar F, Pardo F, Montiel C, et al. Totally laparoscopic Roux-en-Y duct-to-mucosa pancreaticojejunostomy after middle pancreatectomy: a consecutive nine-case series at a single institution. Ann Surg 2008;247:938-44. [Crossref] [PubMed]
- Sa Cunha A, Rault A, Beau C, et al. Laparoscopic central pancreatectomy: single institution experience of 6 patients. Surgery 2007;142:405-9. [Crossref] [PubMed]
- Ronnekleiv-Kelly SM, Javed AA, Weiss MJ. Robotic central pancreatectomy operative video. Asvide 2016;3:329. Available online: http://www.asvide.com/articles/1097
- Kang CM, Kim DH, Lee WJ, et al. Initial experiences using robot-assisted central pancreatectomy with pancreaticogastrostomy: a potential way to advanced laparoscopic pancreatectomy. Surg Endosc 2011;25:1101-6. [Crossref] [PubMed]
- Ikeda S, Matsumoto S, Maeshiro K, et al. Segmental pancreatectomy for the diagnosis and treatment of small lesions in the neck or body of the pancreas. Hepatogastroenterology 1995;42:730-3. [PubMed]
- Balzano G, Zerbi A, Veronesi P, et al. Surgical treatment of benign and borderline neoplasms of the pancreatic body. Dig Surg 2003;20:506-10. [Crossref] [PubMed]
- Shikano T, Nakao A, Kodera Y, et al. Middle pancreatectomy: safety and long-term results. Surgery 2010;147:21-9. [Crossref] [PubMed]
- Adham M, Giunippero A, Hervieu V, et al. Central pancreatectomy: single-center experience of 50 cases. Arch Surg 2008;143:175-80; discussion 180-1. [Crossref] [PubMed]
- Brown KM, Shoup M, Abodeely A, et al. Central pancreatectomy for benign pancreatic lesions. HPB (Oxford) 2006;8:142-7. [Crossref] [PubMed]
- Efron DT, Lillemoe KD, Cameron JL, et al. Central pancreatectomy with pancreaticogastrostomy for benign pancreatic pathology. J Gastrointest Surg 2004;8:532-8. [Crossref] [PubMed]
- Iacono C, Bortolasi L, Serio G. Indications and technique of central pancreatectomy-early and late results. Langenbecks Arch Surg 2005;390:266-71. [Crossref] [PubMed]
- Goudard Y, Gaujoux S, Dokmak S, et al. Reappraisal of central pancreatectomy a 12-year single-center experience. JAMA Surg 2014;149:356-63. [Crossref] [PubMed]
- LaFemina J, Vagefi PA, Warshaw AL, et al. Transgastric pancreaticogastric anastomosis: an alternative operative approach for middle pancreatectomy. Arch Surg 2010;145:476-81. [Crossref] [PubMed]
- Goldstein MJ, Toman J, Chabot JA. Pancreaticogastrostomy: a novel application after central pancreatectomy. J Am Coll Surg 2004;198:871-6. [Crossref] [PubMed]
- Javed AA, Aziz K, Bagante F, et al. Pancreatic Fistula and Delayed Gastric Emptying After Pancreatectomy: Where do We Stand? Indian J Surg 2015;77:409-25. [Crossref] [PubMed]
- Orsenigo E, Baccari P, Bissolotti G, et al. Laparoscopic central pancreatectomy. Am J Surg 2006;191:549-52. [Crossref] [PubMed]
- Ayav A, Bresler L, Brunaud L, et al. Laparoscopic approach for solitary insulinoma: a multicentre study. Langenbecks Arch Surg 2005;390:134-40. [Crossref] [PubMed]
- Müller MW, Friess H, Leitzbach S, et al. Perioperative and follow-up results after central pancreatic head resection (Berne technique) in a consecutive series of patients with chronic pancreatitis. Am J Surg 2008;196:364-72. [Crossref] [PubMed]
- Del Chiaro M, Segersvärd R. The state of the art of robotic pancreatectomy. Biomed Res Int 2014;2014:920492.
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic middle pancreatectomy. J Laparoendosc Adv Surg Tech A 2010;20:135-9. [Crossref] [PubMed]
Cite this article as: Ronnekleiv-Kelly SM, Javed AA, Weiss MJ. Minimally invasive central pancreatectomy and pancreatogastrostomy: current surgical technique and outcomes. J Vis Surg 2016;2:138.


