
High speed robotic mitral valve repair
Introduction
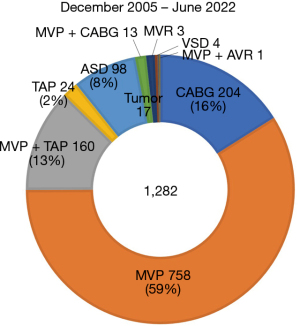
The first robotic mitral valvuloplasty was performed by Carpentier and Mohr in 1998 using an early prototype of the da Vinci Surgical System (1,2) and by Chitwood in 2000 in North America (3). The advantages are smaller, less invasive incisions, less pain, shorter hospital stays, better appearance, faster return to preoperative functional activity levels, and reduced need for blood transfusions. Mitral valve surgery via the lateral approach has the advantage of being minimally invasive, avoiding a median sternotomy, but is reported to require longer operative time than standard techniques, including cardiopulmonary bypass and aortic clamp time (4). Increased operation time can produce results that are contrary to a minimally invasive approach. Starting in 2005 (5), as of July 2022, we have experienced more than 1,280 cases of cardiac surgery using the da Vinci surgical system (Intuitive surgical Inc., Sunnyvale, CA, USA). More than 930 cases of them were mitral valve repair including tricuspid annuloplasty, and the rest were other procedures, such as coronary artery bypass grafting, atrial septal defect closure, cardiac tumor resection, and Maze procedure for atrial fibrillation (6-10) (Figure 1). The advantage of using a surgical robot is that it is possible to perform totally endoscopic surgery via only ports, not thoracotomy. We have performed “keyhole” cardiac surgery with port access only in all but a few patients (Figure 2). We believe mitral valve surgery seems to be the best candidate for this method. We have made several improvements to perform the surgical procedure more safely and quickly, and we would like to introduce our method.
Patients
Nine hundred and twenty-two robotic mitral valve repair cases experienced between December 2005 and July 2022 were included.
The number of patients undergoing concomitant surgery was 30 for tricuspid annuloplasty, 13 for minimally invasive direct coronary artery bypass grafting (MIDCAB) and 1 for aortic valve replacement.
At the beginning, patients over 80 years old, patients with pleural adhesions, and patients with low cardiac function were excluded, but recently robot-assisted surgery has been performed in most cases without any exclusion criteria.
All procedures performed in this work were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Surgical techniques
Cannulation
Under general anesthesia and single-lung ventilation, cardiopulmonary bypass was established with bicaval venous drainage through the right internal jugular vein and the right femoral vein, while arterial perfusion was performed through the right femoral artery. The right internal jugular vein was percutaneously cannulated, and the right femoral artery and vein were cannulated in the right inguinal cut-down.
Totally endoscopic keyhole procedure
For the totally endoscopic mitral valve repair, a right-sided approach was used through four ports: three 10-mm for the robotic camera and the robotic instruments, and another 20-mm service port for the delivery of sutures or the annuloplasty band. We do not use robotic atrial retractor. Insertion of the suction tube through the side of the robotic port minimizes puncture wounds to the skin. In addition, bleeding from port wounds can fall onto the camera or forceps, reducing the quality of the procedure. We use soft tissue retractors on all ports, which help prevent these problems (Figure 3).
Figure 4 knot
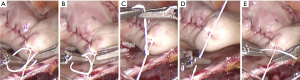
The “Figure 4” technique is an effective ligation method for robotic surgery and has a distinctive merit (11). It can make knot tying dramatically swift and easy because the console surgeon can use two instruments at the same time to ligate the suture, while the patient-side assistant holds one end of the suture to control the tension of the suture (Figure 4).
Transthoracic cardioplegia delivery and aortic-clamp technique
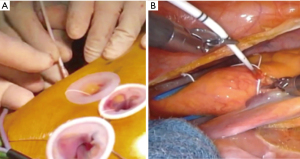
Cardioplegic needle inserted and fixed through the small incision may obstruct the operative view and interfere with the robotic arms in the thoracic cavity. To avoid this, we insert the cardioplegia needle (6 Fr) via the anterior chest wall (12) (Figure 5). There are different ways to cross-clamp the aorta in minimally invasive cardiac surgery. The Chitwood clamp requires an additional small incision. Endovascular balloon clamp technique is cumbersome and needs transesophageal echo guidance. Our technique, introducing the Cygnet flexible aortic clamp (Novare Surgical Systems, Inc., Cupertino, CA, USA) from the service port into the transverse sinus under videoscopic guide, can avoid interference with the robotic arms inside or outside the thoracic cavity, and allows for simple, safe and secure aortic clamping (Figure 6).
V-shape hook
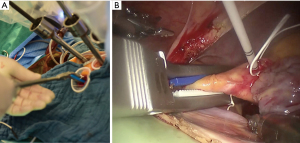
The V-shape hook is our original device: very simple, cheap, and conducive to stabilizing the surgical field (13) (Figure 7). A long straight needle with a V-shaped hook is inserted into the thoracic cavity to tract the atrial wall from outside the body. The device can save the robotic arms. If the V-shape hook is not effective in exposing the left atrial structure, a further original retractor could be used. However, this cannot be published yet due to patent issues. Generally, the EndoWrist atrial retractor (Intuitive surgical Inc.) would be used.
Loop technique
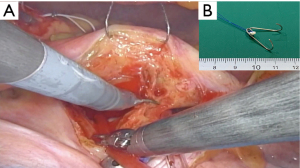
There are many different methods of mitral valve repair, and we have tried a variety of techniques. Now, we prefer “respect rather than resect”. We routinely place polytetrafluoroethylene (PTFE) Neochords (Gore-TexTM-Gore, Inc., Phoenix, AZ, USA) on the redundant part of both anterior and posterior leaflet using the loop technique (Figure 8) (Video 1). The length of the loop is determined intraoperatively by directly measuring the length of the papillary muscle and mitral annulus.
Ring annuloplasty
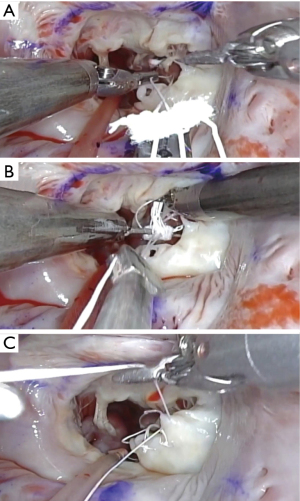
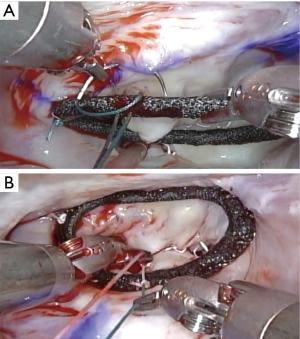
To fix the artificial ring on the mitral valve, we use a simple continuous suture, which can save the time (10–15 minutes) (14) (Figure 9) (Video 1). Cor-KnotTM device (LSI Solutions, Victor, NY, USA) may be useful, but it is relatively expensive.
Shape-memory monofilament suture
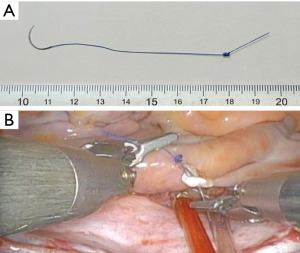
Knot tying is cumbersome and time-consuming in endoscopic surgery. We use a novel shape-memory monofilament suture with a spiral tail developed to speed up suture tying during minimally invasive cardiac surgery (15) (Figure 10). By only passing through the needle and then into the spiral made at the tail of the suture, a hangman’s knot was easily made. It can save the operative time.
Pectus excavatum
Pectus excavatum can be associated with cardiac disorders that also require surgical repair (16). We developed an electrical sternum lifting system (ESLS) which is robust and can be finely adjusted, capable of lifting the sternum by a maximum of 5 to 10 cm. ESLS is employed in the patients whose distance between the sternum and vertebrae is relatively short (less than 80 mm) (17) (Figure 11).
Organization of robotic heart team
To organize the robotic heat team is paramount important to continue successful robotic cardiac surgery (18). We have performed over 1,000 robotic cardiac surgeries with fixed members, including console surgeons and patient-side surgeons. As we piled up our clinical experience, we developed new simple technique and adopted useful technologies and efficiently saved the incisions and instruments, and, as a result, could shorten the operative time without jeopardizing the procedural safety and quality. All of our team members, including surgeons, anesthesiologists, nurses, and perfusionists, have shared not only success but also the lesson we have learned. Such sophisticated teamwork, we believe, would make it possible to perform swift procedures with the highest quality.
Comments
Among cardiac procedures, mitral valve repair to fix mitral insufficiency is best suitable for robotic surgery for two reasons. First, robotic technique can provide surgeons with magnified operative views which allow them to observe closely even the deepest intra-cardiac structure, not only the mitral valve but also mitral subvalvular apparatus. Second, the wrist of robotic-hands is more flexibly mobile than that of human hands, and therefore facilitates complicated maneuvers required to fix mitral valve in endoscopic situation.
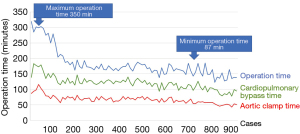
Considering our own experience, the learning curve is expected to be longer than other procedures; our estimate is 150–200 cases. Currently, we can complete mitral repair in 2 hours on average, including 1 hour of aortic clamp. As a matter of fact, every beginner of robotic surgery would spend a longer time to complete the procedure than the time they usually take to perform standard procedures. However, when they do not give up and get over this hard way, they will surely feel the power of robotic surgery; accurate valve repair can be achieved through just keyholes in a shorter time than in the standard procedures. To facilitate the procedure, we have developed some technique and tools that help complete the procedure safely and quickly and have shortened the operative time.
In general, patients who should undergo a median sternotomy are those with aortic valve regurgitation, redo, those with thoracic adhesions, and those concomitant surgery such as coronary artery bypass. However, these patients are now actively performing robotically. For aortic valve regurgitation, retrograde myocardial protection or aortic valve surgery is performed. In Japan, only valve repair is covered by the National Health Insurance, and in principle, robotic surgery is not indicated for valve replacement, which is a major problem. For patients with adhesions, we perform dissection through a small thoracotomy and introduce robotic surgery. Redo cardiac surgery is also possible when adhesions can be safely removed. As for redo patients after robotic surgery which has been performed at our hospital, the initial surgery has been totally endoscopic surgery, so adhesions are rarely present, and robotic redo surgery can be performed easily and safety. In patients with coronary artery stenosis, robotic mitral valve surgery can be performed with simultaneous percutaneous coronary intervention (PCI), minimally invasive cardiac surgery (MICS) coronary artery bypass grafting (CABG) via small thoracotomy, and totally endoscopic coronary artery bypass (TECAB) if not a 3-vessel disease (13).
Conclusions
We have made swift and safe robotic mitral repair possible using simplified technique, new technologies, and making a sophisticated robotic heart team.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Johannes Bonatti) for the series “Robotic Mitral Valve Repair” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-34/coif). The series “Robotic Mitral Valve Repair” was commissioned by the editorial office without any funding or sponsorship. NI serves as an unpaid board member of Japan Robotic Surgery Society from 2014. GW serves as an unpaid board member of Japan Robotic Surgery Society from 2014. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carpentier A, Loulmet D, Aupècle B, et al. Computer assisted open heart surgery. First case operated on with success. C R Acad Sci III 1998;321:437-42. [Crossref] [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Computer-enhanced coronary artery bypass surgery. J Thorac Cardiovasc Surg 1999;117:1212-4. [Crossref] [PubMed]
- Chitwood WR Jr, Nifong LW, Elbeery JE, et al. Robotic mitral valve repair: trapezoidal resection and prosthetic annuloplasty with the da vinci surgical system. J Thorac Cardiovasc Surg 2000;120:1171-2. [Crossref] [PubMed]
- Bush B, Nifong LW, Alwair H, et al. Robotic mitral valve surgery-current status and future directions. Ann Cardiothorac Surg 2013;2:814-7. [PubMed]
- Watanabe G. Successful intracardiac robotic surgery: initial results from Japan. Innovations (Phila) 2010;5:48-50. [Crossref] [PubMed]
- Tarui T, Ishikawa N, Horikawa T, et al. First Major Clinical Outcomes of Totally Endoscopic Robotic Mitral Valve Repair in Japan - A Single-Center Experience. Circ J 2019;83:1668-73. [Crossref] [PubMed]
- Ishikawa N, Watanabe G, Tarui T. No-touch aorta robot-assisted atrial septal defect repair via two ports. Interact Cardiovasc Thorac Surg 2018;26:721-4. [Crossref] [PubMed]
- Ishikawa N, Watanabe G, Tomita S, et al. Robot-assisted minimally invasive direct coronary artery bypass grafting. ThoraCAB. Circ J 2014;78:399-402. [Crossref] [PubMed]
- Ishikawa N, Watanabe G. Ultra-minimally invasive cardiac surgery: robotic surgery and awake CABG. Surg Today 2015;45:1-7. [Crossref] [PubMed]
- Ishikawa N, Watanabe G. Robot-assisted cardiac surgery. Ann Thorac Cardiovasc Surg 2015;21:322-8. [Crossref] [PubMed]
- Ishikawa N, Watanabe G. Figure 4 Knot: Simple Tying Technique for Robotic and Endoscopic Sutures. Innovations (Phila) 2017;12:152-3. [Crossref] [PubMed]
- Watanabe G, Ishikawa N. Alternative method for cardioplegia delivery during totally endoscopic robotic intracardiac surgery. Ann Thorac Surg 2014;98:1129-31. [Crossref] [PubMed]
- Ishikawa N, Watanabe G, Horikawa T, et al. Robot-Assisted Totally Endoscopic Mitral Valve Plasty and Coronary Artery Bypass Grafting. Ann Thorac Surg 2023;115:e93-5. [Crossref] [PubMed]
- Watanabe G, Ishikawa N. Use of barbed suture in robot-assisted mitral valvuloplasty. Ann Thorac Surg 2015;99:343-5. [Crossref] [PubMed]
- Seguchi R, Ishikawa N, Tarui T, et al. A Novel Shape-Memory Monofilament Suture for Minimally Invasive Thoracoscopic Cardiac Surgery. Innovations (Phila) 2019;14:55-9. [Crossref] [PubMed]
- Watanabe G, Matsumoto I, Kiuchi R. Novel sternum lifting technique for robotic internal thoracic artery graft harvesting. Innovations (Phila) 2013;8:76-9. [Crossref] [PubMed]
- Ishikawa N, Watanabe G, Horikawa T, et al. Combined robot-assisted mitral valve plasty and Nuss procedure via small ports. Artif Organs 2021;45:633-6. [Crossref] [PubMed]
- Chitwood WR Jr. Robot-assisted minimally invasive mitral valve surgery. J Vis Surg 2019;5:68. [Crossref]
Cite this article as: Ishikawa N, Watanabe G, Koakutsu T, Horikawa T, Seguchi R, Tomita S, Ohtsuka T. High speed robotic mitral valve repair. J Vis Surg 2023;9:32.


