The benefits and limitations of robotic assisted transhiatal esophagectomy for esophageal cancer
Introduction
Robotic-assisted transhiatal esophagectomy (RATE) was first described by Horgan et al. in 2003 as a minimally invasive alternative to open total esophagectomy (1). In contrast to the classic open technique, minimally invasive total esophagectomy has less morbidity and mortality and results in a shorter length of stay including a drastic reduction in ICU-level care. Other minimally invasive approaches use a combination of laparoscopic and thoracoscopic techniques, which carry these same advantages, however, the learning curve is steep and requires entry into the chest with single lung ventilation for exposure (2,3). The robotic platform offers superior three-dimensional optics, innovative multi-articulated instruments, the ability to perform fine manipulations within the confines of the mediastinum, and intraoperative assessment of graft and anastomotic perfusion with fluorescence angiography, which is why RATE has been the method of choice for total esophagectomy at our institution since 2006.
Operative technique
Our operative team consists of two surgeons, one an expert in minimally invasive and robotic surgery, the other an accomplished surgical oncologist who is well versed in the multiple techniques in total esophagectomy. Both surgeons evaluate the patients pre-operatively which includes endoscopic ultrasound for tumor depth as well as the presence of any lymph node metastasis. A PET-CT is also obtained to evaluate for metastatic disease. In overweight or obese patients, a 2 to 4 weeks bariatric liquid diet is prescribed in an attempt to reduce visceral and mediastinal fat and aid with visualization. Patients with locally advanced disease (T2 or greater or node positive) complete a course of chemoradiation prior surgery through our comprehensive cancer center. All patients receive an upper GI endoscopy to once again directly visualize the lesion and assess for disease progression before proceeding with the case. Barring unanticipated progression of disease, the case is continued by the endoscopic injection of 200 units of BOTOX circumferentially into the pylorus to aid with postoperative gastric emptying (4). Pyloroplasty is not routinely performed.
The patient is positioned split leg on a beanbag device with heavy padded leg straps, which allows us to operate safely in a steep Trendelenburg position. Port sites are judiciously placed in a position that is optimal for both the laparoscopic and the robotic segments of the case. A 12-mm trocar is used for the robotic camera port and is positioned in the left upper quadrant just to the left of midline. Two 8-mm robotic trocars are placed in each the left and right upper quadrants which double as working ports for the laparoscopic portion of the case. A 10-mm assistant port is placed in the left lateral position, a second, 5 mm assistant port placed in the left mid-abdomen, and a Nathanson liver retractor is placed to aid with visualization and exposure (Figure 1).

We begin the procedure laparoscopically by mobilizing the greater curvature of the stomach and taking down the short gastric vessels with a laparoscopic ultrasonic scalpel. Care is taken to ensure the right gastroepiploic artery and tributaries are avoided to prevent ischemia to the tubularized gastric graft. The dissection is continued until the left crus encountered at which point the esophagus is dissected circumferentially off of the crura of the diaphragm and encircled with a Penrose drain, which is used by the assistant to aid with retraction. The dissection is continued along the lesser curvature until the left gastric artery is identified and divided with an endoscopic vascular stapler. The robot platform is then docked, coming in at a 45-degree angle over the patients left shoulder. Starting the case laparoscopically allows the surgeon to begin the dissection while the surgical technician and circulating nurse set up and drape the robot, maximizing time and efficiency in the operating room.
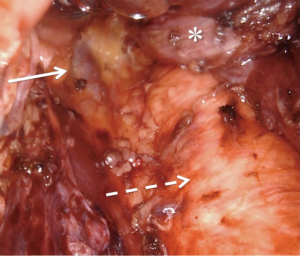
The primary surgeon then continues the case at the robotic console. The assisting surgeon remains scrubbed in as the bedside assistant to provide critical traction of the esophagus. The circumferential dissection of the esophagus proceeds proximally with care to include all periesophageal tissue and lymph node-containing fat. In the obese patient, visualization and exposure are considerably improved when the patient has been adherent to the pre-operative bariatric liquid diet. Many patients receive neoadjuvant chemoradiation, which can cause inflammation and scarring, making the plane of dissection between the esophagus and pleura difficult to discern. In the event that the pleura are entered, it is immediately repaired with either a clip or a running simple suture. We do not routinely place chest tubes, even when the pleura are entered, as carbon dioxide pneumothoraces without parenchymal lung injury are self-limited and hemodynamically insignificant in nearly all cases. The dissection is carried as proximal as possible along the esophagus taking full advantage of the multi-articulating instruments, tremor reduction, and three-dimensional visualization that the robotic platform offers while operating within the confines of the mediastinum. At the completion of the esophageal dissection the azygos vein will be clearly visualized to the right with the aorta to the left (Figure 2).
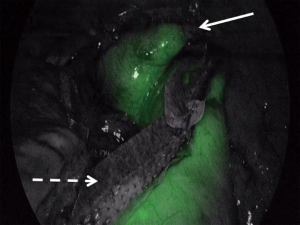
Upon completion of the esophageal dissection the robotic portion of the case is completed when the esophagus is fully dissected. The robot is undocked and the patient cart is positioned away from the operating field. The surgical oncology team begins the left neck dissection to access the cervical esophagus while the minimally invasive team prepares for the laparoscopic creation of the neoesophagus. Care is taken to preserve the recurrent laryngeal nerve during the cervical dissection. While the neck dissection is underway, the minimally invasive team re-insufflates the abdomen and laparoscopically creates the tubularized gastric conduit that will become the neoesophagus. The stomach is divided along the lesser curvature with a linear endoscopic stapler that is reinforced with a layer of polyglycolic acid:trimethylene carbonate (PGA:TMC), a synthetic bioabsorbable copolymer. When completed, the tube measures approximately 6 cm in width. At this point the perfusion of the newly created tubularized gastric graft can be assessed with indocyanine green (ICG) fluorescence angiography. The technique involves the intravenous injection of 7.5 mg of ICG and assessment of the microvascular perfusion along the length of the graft with one of several commercially available laparoscopes that have fluorescence capability (Figure 3). Special attention is paid to the proximal tip of the tubularized gastric graft. Any poorly perfused areas can be visualized and avoided during the creation of the cervical esophagogastric anastomosis.
The next step is to position the neoesophagus in the mediastinum and create a tension free cervical esophagogastric anastomosis. The fundus of the graft is sutured to the distal end of the resected specimen to assist with optimal positioning of the gastric graft without twisting or kinking (Figure 4). The surgical oncologist pulls the fully mobilized esophageal specimen through the cervical neck incision as the minimally invasive surgeon visualizes the specimen and tubularized gastric graft pass through the hiatus from below. The proximal aspect of the esophagus is divided and the side-to-side esophagogastric anastomosis is created and then oversewn with interrupted silk sutures. A Jackson-Pratt drain is left in place in the cervical neck incision and maintained until that patient is tolerating an oral diet without an increase in drain output.
Most patients are extubated in the operating room and transferred to either the ICU or the step-down unit at the surgeon’s discretion for post-operative care. We do not routinely place jejunostomy feeding tubes. All patients complete an esophagogram on postoperative day 3, and if no extravasation is noted the patient is advanced to a liquid diet. Routine follow up with scheduled imaging studies is arranged for each patient to monitor for local recurrence and metastatic disease (Figure 5).

Discussion
Minimally invasive esophagectomy has been shown to have perioperative outcomes that are superior to the open approach without compromising survival (6). The physiologic demands of an open procedure significantly outweigh those of a thoracoscopic and/or laparoscopic approach, which is why we see a consistently shorter length of stay in patients who have had the minimally invasive approach. Further, fewer resources are utilized in the postoperative management of these patients since they typically require far fewer days in the intensive care unit and sometimes are even transferred to the step-down unit on the same day of surgery.
As is true for open esophagectomy, there are a variety of minimally invasive techniques available for esophagectomy. Regardless of the minimally invasive technique selected the dissection is challenging to learn and the robotic platform offers a significant teaching advantage when two consoles are available. The experienced surgeon can take a junior surgeon through the mediastinal dissection using on-screen visual cues to guide them through the often times challenging surgical plane, and take over when appropriate. Instruction during laparoscopic/thoracoscopic cases is reliant on verbal instruction, which can be considerably less effective. The learning curve for robotic over purely laparoscopic/thoracoscopic esophagectomy may therefore be lower.
The robotic platform has a number of features that are major assets during RATE. Because there are two closely spaced cameras, the operative field is displayed in three-dimension on the robotic surgeon’s console, which gives the surgeon the added benefit of depth perception for superior surgical navigation. Control of the robotic instruments is also superior to laparoscopic or open for a number of reasons. First, the instruments have multi-articulating arms so they have increased rotational freedom over standard laparoscopic tools. As for control of the instruments, there is tremor reduction and adjustable motion scaling which allows for greater precision. There is also an improvement in ergonomics for the surgeon since the controls can be adjusted at any time back to the optimal operating position, and when the surgeon releases the controls the robotic platform will hold the position of the instruments steadily in place. All of these features make the robotic platform an outstanding option for performing fine manipulations in small spaces like the mediastinum. Further, the robot has built-in ICG fluorescent angiography technology for localization and preservation of the right gastroepiploic artery, and to check perfusion of the gastric conduit.
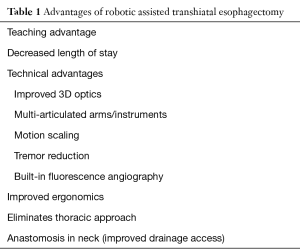
The robotic-assisted transhiatal approach avoids the need for routine entry into the pleural cavity, which itself has numerous advantages. Thoracoscopic approaches require right lung collapse and single lung ventilation, which can be problematic in patients with underlying lung disease. Further, postoperative pain and discomfort may be reduced in patients where thoracic access is avoided and chest tubes are not routinely placed. And finally, by placing the anastomosis high in the cervical esophagus, any potential leak will typically drain to the skin incision rather than into the chest and mediastinum as is the case with a mid-esophageal anastomosis. A full summary of the advantages to RATE can be seen in Table 1.
Full table
The major criticism of this approach is that fewer lymph nodes are retrieved than with a thoracic exposure where one can perform a formal lymph node dissection. The National Comprehensive Cancer Network guidelines recommend retrieving at least 15 nodes to appropriately stage a patient without neoadjuvant chemoradiation (7). Institutions performing RATE report obtaining at least this many nodes in published case series (8,9). Some series report increased survival with en-bloc dissections where more lymph nodes are presumably obtained (10), but a recent meta-analysis with over 1,300 patients undergoing a minimally invasive approach showed no difference (6). The robotic approach is also not ideal for large or bulky tumors, and should not be used in there is concern for involvement of other mediastinal structures.
An additional criticism is the need to place the anastomosis proximally in the neck as opposed to in the mediastinum. When compared to mediastinal anastomoses, cervical anastomoses are theoretically at higher risk for ischemia and tension due to the greater distance that the conduit must reach. Additionally, pulling the conduit up through the thoracic inlet can cause some degree of venous congestion that may impact anastomotic healing. For these reasons, some feel that the cervical anastomosis is at higher risk for leak or stricture than mediastinal anastomoses and prefer the latter. The flip side to this argument is that a cervical anastomotic leak much less morbid than a mediastinal anastomotic leak and can usually be managed conservatively with parenteral or distal enteral nutrition and continued drainage with the operatively placed drain.
A final drawback to this technique is the cost of using the robot and whether that cost is recouped by the benefits listed above. One obvious pitfall is in the case where the surgeon decides it is not safe to proceed robotically and the case is converted to open. In this scenario the case sustains all of the cost of a robotic procedure without any of the benefit. Additionally, as many of these cases are performed in busy tertiary care centers there may be high demand for block time for robotic cases. And finally, whenever there are two operating surgeons there can be the challenge of scheduling both surgeons for the same case. A summary of the drawbacks of RATE can be found in Table 2.
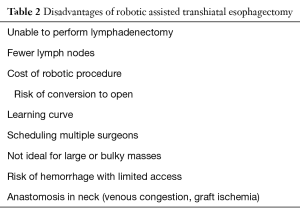
Full table
Patient selection for this technique is critical for it to be successful and to minimize the need for conversion to open. Patient body habitus is an important consideration. Whether open or robotic-assisted, the transhiatal approach is challenging in very tall patients or those with a long thorax. It can be very difficult to achieve communication between the proximal and distal dissection planes in these patients and access to the right chest may be required for a complete dissection. Similarly, patients with GEJ tumors extending down into the gastric cardia will require resection of a portion of the proximal stomach and the resulting gastric conduit may not reach the neck. In these cases the Ivor-Lewis technique with mediastinal anastomosis is required. A final consideration before embarking on RATE is surgeon experience and preparation of an appropriate operative team. It is critical to have an experienced minimally-invasive surgeon and surgical oncologist for this technique to be performed safely. Dissection in the mediastinum with the robot docked has the potential for significant bleeding with minimal exposure. A plan for rapid availability of blood products and rapid conversion to open must be in place and be well-understood by all team members.
Conclusions
In conclusion, RATE is a superior operation to the conventional open technique because it yields less perioperative morbidity while maintaining oncologic efficacy. The robotic platform offers numerous technical advantages over other minimally invasive techniques and can achieve a complete resection without entering the thoracic cavity. The major disadvantage of this technique is the inability to perform a formal lymphadenectomy, but this does not appear to have a deleterious effect on long-term oncologic outcomes.
As with open approaches, the choice of minimally-invasive technique for esophagectomy is likely to be dependent on surgeon experience and preference. RATE is an excellent option for well selected patients such as those with mid-to-distal esophageal tumors not invading adjacent structures and not extending into the proximal stomach, and those with underlying lung disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Horgan S, Berger RA, Elli EF, et al. Robotic-assisted minimally invasive transhiatal esophagectomy. Am Surg 2003;69:624-6. [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [PubMed]
- Montenovo MI, Chambers K, Pellegrini CA, et al. Outcomes of laparoscopic-assisted transhiatal esophagectomy for adenocarcinoma of the esophagus and esophago-gastric junction. Dis Esophagus 2011;24:430-6. [Crossref] [PubMed]
- Fuchs HF, Broderick RC, Harnsberger CR, et al. Intraoperative Endoscopic Botox Injection During Total Esophagectomy Prevents the Need for Pyloromyotomy or Dilatation. J Laparoendosc Adv Surg Tech A 2016;26:433-8. [Crossref] [PubMed]
- DeLong JC, Kelly KJ, Bouvet M, et al. Supplemental video of key steps selected to demonstrate the advantages of robotic assisted transhiatal esophagectomy. Asvide 2016;3:369. Available online: http://www.asvide.com/articles/1138
- Yerokun BA, Sun Z, Jeffrey Yang CF, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [PubMed]
- Coker AM, Barajas-Gamboa JS, Cheverie J, et al. Outcomes of robotic-assisted transhiatal esophagectomy for esophageal cancer after neoadjuvant chemoradiation. J Laparoendosc Adv Surg Tech A 2014;24:89-94. [Crossref] [PubMed]
- Dunn DH, Johnson EM, Morphew JA, et al. Robot-assisted transhiatal esophagectomy: a 3-year single-center experience. Dis Esophagus 2013;26:159-66. [Crossref] [PubMed]
- Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg 2001;234:581-7. [Crossref] [PubMed]
Cite this article as: DeLong JC, Kelly KJ, Jacobsen GR, Sandler BJ, Horgan S, Bouvet M. The benefits and limitations of robotic assisted transhiatal esophagectomy for esophageal cancer. J Vis Surg 2016;2:156.


