Resection of a micronodular thymoma with lymphoid stroma achieved by robot-assisted thoracic surgery (RATS): a case report
Highlight box
Key findings
• Micronodular thymoma with lymphoid stroma (MTWLS) is a very rare entity and thus establishing a proper diagnosis is crucial.
What is known and what is new?
• The entity of MTWLS is known since around 1990. We present a case with our therapeutic approach of robot-assisted thoracic surgery (RATS) resection.
What is the implication, and what should change now?
• MTWLS should be further investigated especially the therapy with RATS resection.
Introduction
Thymoma is a rare potentially malignant neoplasm in adults. The reported incidence in the population varies from 2.2 to 2.6 per million per year (1). Histologically the neoplasm originates from the epithelial cells of the thymus (2). The World Health Organization (WHO) classified different types of thymoma, known as A, AB, B1, B2 and B3 (3). Some other rare subtypes are present including micronodular thymoma with lymphoid stroma (MTWLS), lipofibroadenoma, sclerosing thymoma, microscopic thymoma and metaplastic thymoma (3). MTWLS occur in only about 1% of all thymoma cases (2). Due to its low incidence, we have limited understanding of its behaviour. MTWLS is more common in males, with a male-to-female ratio of 1.3:1 (4). The reported mean patient age is 64 years, with a range from 41 to 80 (5). Most patients are asymptomatic, and the tumour is an incidental radiological finding. MTWLS has a similar frequency of paraneoplastic syndrome like other types of thymoma (6). Clinical guidelines and clear therapeutic regimens are lacking. In this case report, we present our therapeutic approach to a case of MTWLS. We present this case in accordance with the CARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-16/rc).
Case presentation
Clinical history
A 83-year-old woman was referred to our division of general thoracic surgery with an undiagnosed anterior mediastinal mass.
The patient reported chest pain occurring especially during the night. Further investigation of the heart with echocardiography showed a normal systolic ejection fraction and the ergometry showed a limited capacity (82% of desired value) with increased events of ventricular extrasystoles. Myastenia gravis or other autoimmune disease were absent.
Radiological findings
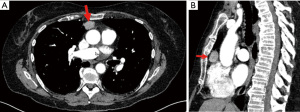
A coronary computed tomography was performed and showed normal coronary arteries without any plaques but as an incidental finding a mass in the anterior mediastinum. On performing computed tomography (CT) scan of the chest, an anterior mediastinal mass was found in the presumed Masaoka Stage I. The mass was located cranial to the right atrium and ventral to the aortic root paramedial on the right side with a native density value of 40 Hounsfield units (HU) and of 92 HU after contrast administration. The size of the mass was 22 mm × 16 mm × 24 mm (Figure 1). CT scan showed no enlarged lymph nodes or other suspect lesions in the thorax. The mass increased in size when compared to the size reported in a previous CT scan done 5 years earlier 9 mm × 8 mm × 11 mm.
Surgical technique
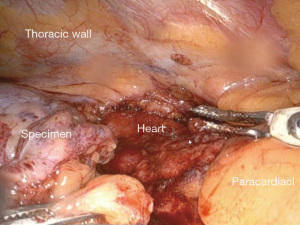
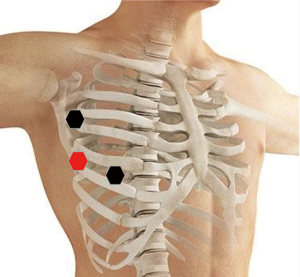
After getting an informed consent, right sided robot-assisted thoracic surgery (RATS) thymectomy was decided due to its minimal invasive approach. In our institution criteria for RATS thymectomy are according to The European Society for Medical Oncology (ESMO) guidelines and tumour size less than 8 cm, and other entity than thymus carcinoma. If ever possible we are deciding for a minimal invasive approach. In our institution we are using Intuitive DaVinci Xi system. The patient was placed in a supine position on the operating table. We positioned three ports in single lung ventilation with CO2 insufflation with a pressure value of 12mmHg. Three ports were used in our procedure. The camera port was placed in the fifth intercostal space in the anterior axillary line, the working ports were placed in the deltopectoral groove on the right side in the third intercostal space and in the medioclavicular line in the fifth intercostal space (Figure 2). During intrathoracic inspection no adhesions were observed, and the mass could easily be identified in the anterior mediastinum in a retrosternal position. We used bipolar Maryland forceps and fenestrated bipolar forceps for preparation. We opened the parietal pleura ventrally to the phrenic nerve on the right side and performed en bloc resection of the pericardial fat and tumour tissue including the thymus horns. The mass could easily be dissected without any adhesions and with maintaining the capsule unimpaired. The references where the Ligamentum thyroideus cranial, the diaphragm caudal and the right phrenic nerve according to ESMO guidelines. The left pleura space was not damaged during the procedure and the left phrenic nerve not identified due to the right sided location of the mass. The mass was packed in a bag and removed through the ventral access in the fifth intercostal space. Control of haemostasis was performed, a chest tube was inserted, the right lung was inflated again, and the wounds were closed. The intraoperative course was uneventful with minimal blood loss (Figures 3,4). The resected specimen was sent to our pathology department for analysis.
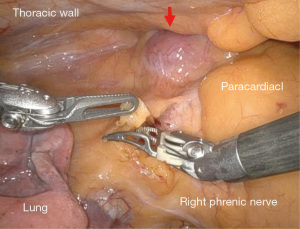
Patient course after surgery
Postoperative course was uneventful. The patient was discharged at the third postoperative day. A clinical and radiological follow up in our outpatient clinic two weeks after the operation showed further no complications and a patient in good clinical shape without any symptoms.
Histopathological features
On macroscopic examination the tumour was encapsuled with a size of 30 mm × 30 mm × 15 mm and located in the specimen with surrounding fatty tissue. On microscopic examination the tumour was composed of multiple small, partly coalescing nodules of bland spindle cells with indistinct nucleoli and minimal mitotic activity separated by an abundant lymphoid stroma (Figure 5A,5B). Some parts or the tumour showed macroscopic transcapsular invasion leading to a pathological Masaoka stage II. The tumour cells were positive for CK19 (Figure 5C) and p40 (Figure 5D). The lymphoid stroma consisted primarily of mature CD20+ B cells and CD3+/TdT− T cells with a smaller population of immature TdT+ thymocytes (Figure 5E) without the meshwork of CK19+ epithelial cells characteristic of type AB thymoma (Figure 5C). The tumour nodules also contained abundant CD1a+/langerin+ Langerhans cells (Figure 5F). Details of microscopic marker are shown in Table 1.
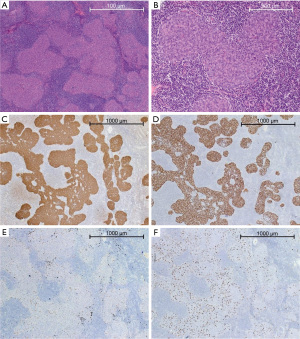
Table 1
| Microscopic marker | Positive | Negative |
|---|---|---|
| BS5 | + | |
| p40 | + | |
| CK19 | + | |
| CD1a | + | |
| Langerin | + | |
| CD99 | + | |
| CD3 | + | |
| CD20 | + | |
| CD79a | + | |
| TdT | − | |
| PAX-8 | − |
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Follow up
We discussed the case at our interdisciplinary board for thoracic malignancies. The panel decided for clinical follow up at the family physician.
Discussion
MTWLS most often occurs in young male patients and is asymptomatic at the time of diagnosis. In preoperative imaging MTWLS it is difficult to differentiate from other subtypes as similar density in CT scans are observed. The chest pain the patient had is unspecific and not related to a specific subtype of thymoma. According to the current literature the pathological stage I or II, according to the WHO classification, have been reported in 95% of cases (7). Pathological findings in our case fit to the literature. Histologically, MTWLS consists of multiple small nests of tumour cells separated by an abundant lymphoid stroma. The tumour nests are positive for CK19 and p40, and the stroma consists of mature lymphocytes with only a minor population of TdT+ thymocytes (3,5). The tumour nests contain a population of Langerhans cells, and it has been suggested that the indolent behaviour of the tumour may be attributable to the immune response induced by these cells (8). The main differential diagnoses are type AB thymoma and micronodular thymic carcinoma with lymphoid hyperplasia. Type AB thymoma consists of a lymphocyte-poor spindle cell component similar type A lymphoma and a lymphocyte-rich component similar type B lymphoma with a significant population of TdT+ thymocytes and a meshwork of CK19+ epithelial cells (3). Micronodular thymic carcinoma with lymphoid hyperplasia is even less common than MTWLS and exhibits evident features of malignancy such as cytologic atypia and increased mitotic activity (9).
There has been no report in the literature of recurrence, distant metastasis, or tumour-related death if a MTWLS is completely resected. There is one case in the literature where local recurrence happened 10 years after primary resection of an incomplete resected MTWLS (10). In our case with R0 resection of the tumour we do not expect any recurrence thus no further treatment is necessary. In the clinical follow up until know no science for recurrence were observed. Our case also illustrates that RATS approach for thymectomy is a safe technique to perform surgical resection. In the current literature there were no cases of MTWLS described which were resected by RATS.
Recent studies have reported comparable oncological outcomes in RATS versus transsternal thymectomy with more favourable outcomes in RATS group regarding length of hospital stay, intraoperative blood loss, operative time, and chest tube duration (11,12). RATS was associated with less blood loss in operation, lower volume of drainage, fewer postoperative pleural drainage days, shorter postoperative hospital stay, and fewer postoperative complications compared to VATS (13). There was no significant difference in operative time and patients with or without myasthenia gravis (13).
Limitations are the small number of cases. We could present only one case of this rare entity at our institution. For the future larger studies are needed to evaluate if RATS resection of MTWLS is a safe and feasible procedure.
Conclusions
In conclusion MTWLS is a very rare entity and thus establishing a proper diagnosis is crucial. We demonstrate one case of this rare entity with our approach of RATS resection. Further investigations are needed to find out if RATS resection of a MTWLS is safe procedure and if it should be considered as standard procedure in this type of tumours. For this more data with long term follow up are needed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Gregor J. Kocher) for the series “Robotic Thoracic Surgery: Established Procedures & Current Trends” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-16/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-16/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-16/coif). The series “Robotic Thoracic Surgery: Established Procedures & Current Trends” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gadalla SM, Rajan A, Pfeiffer R, et al. A population-based assessment of mortality and morbidity patterns among patients with thymoma. Int J Cancer 2011;128:2688-94. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon: IARC. 2015; 205-206.
- Travis WD, Brambilla E, Burke AP, et al. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Mneimneh WS, Gökmen-Polar Y, Kesler KA, et al. Micronodular thymic neoplasms: case series and literature review with emphasis on the spectrum of differentiation. Mod Pathol 2015;28:1415-27. [Crossref] [PubMed]
- Suster S, Moran CA. Micronodular thymoma with lymphoid B-cell hyperplasia: clinicopathologic and immunohistochemical study of eighteen cases of a distinctive morphologic variant of thymic epithelial neoplasm. Am J Surg Pathol 1999;23:955-62. [Crossref] [PubMed]
- Tateyama H, Girard N, Marx A, et al. Micronodular thymoma with lymphoid stroma. In: Travis WD, Brambilla E, Nicholson AG, editors. WHO classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: World Health Organization; 2015. p. 205-6.
- Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015;10:367-72. [Crossref] [PubMed]
- Ishikawa Y, Tateyama H, Yoshida M, et al. Micronodular thymoma with lymphoid stroma: an immunohistochemical study of the distribution of Langerhans cells and mature dendritic cells in six patients. Histopathology 2015;66:300-7. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Micronodular thymic carcinoma with lymphoid hyperplasia: a clinicopathological and immunohistochemical study of five cases. Mod Pathol 2012;25:993-9. [Crossref] [PubMed]
- Kaminuma Y, Tanahashi M, Yukiue H, et al. Micronodular thymoma with lymphoid stroma diagnosed 10 years after the first operation: a case report. J Med Case Rep 2019;13:69. [Crossref] [PubMed]
- Azenha LF, Deckarm R, Minervini F, et al. Robotic vs. Transsternal Thymectomy: A Single Center Experience over 10 Years. J Clin Med 2021;10:4991. [Crossref] [PubMed]
- Agatsuma H, Yoshida K, Yoshino I, et al. Video-Assisted Thoracic Surgery Thymectomy Versus Sternotomy Thymectomy in Patients With Thymoma. Ann Thorac Surg 2017;104:1047-53. [Crossref] [PubMed]
- Shen C, Li J, Li J, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for treatment of patients with thymoma: A systematic review and meta-analysis. Thorac Cancer 2022;13:151-61. [Crossref] [PubMed]
Cite this article as: Schnider M, Svantesson T, Azenha LF, Kestenholz P, Minervini F. Resection of a micronodular thymoma with lymphoid stroma achieved by robot-assisted thoracic surgery (RATS): a case report. J Vis Surg 2023;9:44.