
Valve-in-valve transcatheter aortic valve replacement for the degenerated rapid deployment PercevalTM prosthesis: technical considerations
Introduction
Bioprosthetic surgical aortic valve failure requiring reintervention is a frequent clinical problem with event rates ranging from 5% to 20% at 10-year follow-up, depending on the valve type (1,2). Most recent bioprostheses present novel tissue treatment solutions primarily targeting a reduction in leaflet calcification leading to improved valve durability (3,4). Rapid deployment aortic valve prostheses [so-called “sutureless valves” (SV)] by design privilege a favorable hemodynamic profile, in part because valve anchoring relies on self-expanding properties of the valve frame at the level of the aortic annulus, without the need of multiple sutures on a suture ring. Therefore, the use of SV makes surgery faster and less invasive, on top of showing excellent early and late outcome, and a very low incidence of structural valve degeneration (SVD), even in challenging patients (5,6).
While redo cardiac surgery remains a valid option for symptomatic patients with bioprosthetic valve failure, valve-in-valve transcatheter aortic valve replacement (ViV TAVR) has become the preferred approach because of its high procedural success rate (>90%) and the inherent risk of redo-surgery in an often-elderly population (7). However, careful consideration of anatomical characteristics and features of the surgical prosthesis is needed to assess feasibility of the procedure. A systematic approach to planning of ViV TAVR has been described elsewhere, with meticulous attention to avoid coronary obstruction, device malpositioning and high residual transprosthetic gradients (8). We here systematically describe practical considerations for the transcatheter treatment of failing PercevalTM SV (Corcym srl, Milan, Italy).
All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying videos.
PercevalTM as a receptor for a transcatheter heart valve (THV)
SV have been designed to combine best of both worlds, incorporating decades of surgical bioprosthetic valve technology into THV-like stent frames, hereby improving hemodynamic performance and simplifying valve fixation. However, in case of SVD of the PercevalTM SV, redo cardiac surgery can be challenging, with a risk of annular injury and the need for root replacement. This is particularly relevant when use is considered in young patients, who may require multiple future procedures.
While initially not designed to serve as a docking station for transcatheter valves in case of SVD, PercevalTM combines several features that are of particular interest for a safe performance of ViV procedures. First, the nitinol radiopaque stent frame ensures clear visibility for imaging during preprocedural computerized tomography (CT) planning and fluoroscopy-guided valve implantation. Several essential landmarks can easily be identified on the stent frame, providing the operator complete control during the ViV procedure, having a precise indication of the position of the transcatheter valve in relation to the PercevalTM valve (Figure 1).
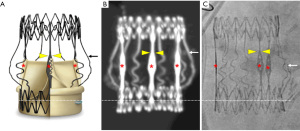
Second, PercevalTM provides for each size model [small (S), medium (M), large (L), and extra large (XL)] an even circumferential expansion of 2.5 mm above nominal size at the level of the inflow ring, allowing for greater compatibility with THV models and sizes, and translating into hemodynamic advantages (9).
Third, by design of the receptor valve, ViV TAVR in PercevalTM carries a minimized risk of coronary obstruction and of sinus sequestration. A virtual THV to coronary (VTC) distance (i.e., the horizontal distance between the coronary ostium and the THV frame on the preprocedural CT) of less than 4 mm has been identified as an independent predictor of coronary obstruction (10). In case of ViV TAVR in PercevalTM, the VTC corresponds to the space between the sinusoidal struts and the open leaflets, a distance that varies between 5 mm for the PercevalTM S to 6.5 mm for PercevalTM XL and is not influenced by the THV frame (Figure 2A), except in case of gross oversizing of the THV. Similarly, by design, the PercevalTM leaflets do not touch the sinotubular junction when reaching their highest point in open position, hereby avoiding sinus sequestration, and maintaining coronary patency (Figure 2B).

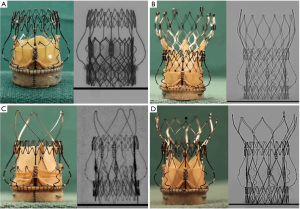
As such, PercevalTM represents an ideal docking station for most currently available transcatheter valves in case of SVD (Figure 3). Depending on the size of the receiver valve, and after cautious review of available CT images to assess true internal diameters and anatomic peculiarities, the size of the corresponding THV can be selected using the ViV aortic app [UBQO Ltd. (London, UK) and Dr. Vinayak Bapat (St. Thomas Hospital, London, UK)]. Ideally, a CT-based computer simulation of the implant should be performed to assess the interaction of the THV with the SV and with the surrounding anatomy. It remains important to consider limitations of some THV frames, when used in PercevalTM S. More specifically, the Acurate NeoTM (Boston Scientific, Marlborough, MA, USA) should be used with caution in these circumstances, since implantation may result in incomplete expansion of the upper crown and a higher transprosthetic gradient. Similarly, the larger and longer frame at the outflow side of other self-expanding THVs requires sufficient space to allow for maximal valve expansion of this upper segment, to improve hemodynamic outcome. Finally, depending on the specific patient context, transcatheter valves with intra-annular leaflet position or low commissural height and large open cells may be preferable in terms of coronary access after THV-in-SV. In this respect, general principles for the choice of a THV-in-THV equally apply to THV-in-SV with a stented frame (11).
Procedural tips and tricks
Procedural success of ViV TAVR in PercevalTM depends essentially on meticulous preprocedural work-up. Once the appropriate valve and size have been selected, the implant procedure itself is usually straight forward, with short procedure times and limited use of contrast. Care should be taken during retrograde wire crossing to avoid wire entanglement or “fausse route” outside the stented frame of PercevalTM, especially in patients with wide sinutubular junction. In these patients the outflow of PercevalTM typically is not apposed to the aortic wall, leaving a space for the crossing wire. In case of doubt, wire trajectory should be checked in several fluoroscopic projections, and free movement of the pigtail catheter during wire exchange should be confirmed.
As with other ViV procedures, we usually don’t recommend balloon predilatation of the degenerated bioprosthesis, as it may increase the risk for stroke and severe valve regurgitation. However, predilatation may be indicated in cases where severe calcification is observed on the preprocedural CT, especially to avoid severe underexpansion of a self-expanding prosthesis. In such cases, the THV should be prepared beforehand and ready to go for salvage of catastrophic acute aortic regurgitation.
Valve positioning is entirely guided by essential radiopaque landmarks on the PercevalTM. The golden rule here is proper alignment of the inflow of both the receptor and new implant. For self-expanding valves with a bottom-to-top expansion, such as EvolutTM or PorticoTM, a perfect overlap of both inflow rings can be targeted and adjusted during progressive valve deployment (Figure 4, left). When a balloon-expandable Sapien 3TM valve is used, one should account for the shortening of the valve frame at the inflow side. Therefore, the inflow side of the unexpanded SapienTM is typically protruding into the left ventricular outflow tract, while the outflow is aligned with the column tapers of PercevalTM. Such positioning accounts for the foreshortening of the valve during expansion and allows perfect alignment of both valve frames upon full expansion (Figure 4, right). Representative procedural steps of EvolutTM and SapienTM in PercevalTM implantation are presented in Videos 1,2.

Case experience
The versatility of PercevalTM favors the use of this valve in a wide variety of clinical situations, especially in elderly patients. In our experience, despite the intermediate risk profile of the treated population, long-term clinical outcomes were good, with incidence rates of endocarditis of 0.46% and severe SVD of 0.54% per patient year (15 patients in 784 implants) (5). Of these 15 patients with pure SVD, only 9 were scheduled for and underwent successful ViV TAVR. Patient and procedural characteristics are presented in Table 1. Most received their PercevalTM before we adapted our sizing strategy in 2017 (6), and none of them were treated with Perceval PlusTM, since this valve with FREE tissue treatment was only introduced in our practice in 2020 (4). None of the patients underwent predilation of the degenerated PercevalTM. Postdilation was performed in patients receiving EvolutTM only, due to visible underexpansion in one, and high invasive peak-to-peak transprosthetic gradient in the other (45 mmHg). In these patients, postdilation resulted in residual invasive peak-to-peak transprosthetic gradients of 2 and 20 mmHg, respectively. The patients in Table 1 are currently undergoing serial clinical and echocardiographic follow-up at 1 and 6 months, and yearly after THV-in-SV, and all patients present transprosthetic gradients similar to the immediate postprocedural result and absent or trivial aortic regurgitation. The longest available follow-up to date is 3 years after the ViV procedure.
Table 1
| Gender, age† (years) | PercevalTM size | TPG post-implant (mmHg), peak/mean | Time interval (years) | Before ViV procedure | EF (%) | TAVR device | After ViV procedure | ||
|---|---|---|---|---|---|---|---|---|---|
| TPG (mmHg), peak/mean | AR grade | TPG (mmHg), peak/mean | AR grade | ||||||
| F, 74 | L | 32/15 | 5.0 | 61/37 | Severe | 30 | Sapien 3TM, 26 mm | 21/13 | None |
| F, 83 | L | 20/10 | 5.8 | 114/68 | Mild | 62 | Sapien 3TM, 23 mm | 31/18 | None |
| F, 75 | L | 20/13 | 4.6 | 95/48 | None | 58 | Sapien 3TM, 23 mm | 26/17 | None |
| M, 82 | M | 23/13 | 3.6 | 89/52 | Mild | 55 | Sapien 3TM, 23 mm | 21/12 | None |
| M, 76 | L | 23/14 | 7.6 | 59/34 | None | 60 | Sapien 3TM, 26 mm | 26/14 | None |
| F, 80 | M | 27/19 | 6.5 | 67/44 | Moderate | 55 | Evolut RTM, 26 mm | 19/11 | None |
| F, 78 | M | 23/15 | 4.9 | 72/49 | Moderate | 50 | Sapien 3TM, 23 mm | 50/28 | None |
| F, 79 | S | 19/13 | 4.5 | 51/30 | Moderate | 32 | Sapien 3TM, 23 mm | 17/8 | None |
| M, 82 | XL | 20/12 | 6.1 | 91/52 | None | 60 | Evolut RTM, 29 mm | 32/17 | None |
†, age refers to the age at ViV TAVR. TPG, transprosthetic gradient; ViV, valve-in-valve; AR, aortic regurgitation; EF, ejection fraction; TAVR, transcatheter aortic valve replacement; F, female; L, large; M (in gender), male; M (in size), medium; S, small; XL, extra large.
Conclusions
While SVD is rare in large patient series undergoing SV implantation, PercevalTM appears to be ideally suited to receive a transcatheter valve, without compromising future valve hemodynamics and coronary patency, provided meticulous valve selection is performed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Aleksander Dokollari, Basel Ramlawi, and Gianluigi Bisleri) for the series “Sutureless Valves” published in Journal of Visualized Surgery. The article has undergone external peer review.
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-28/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-28/coif). The series “Sutureless Valves” was commissioned by the editorial office without any funding or sponsorship. CD receives minor speaker fees from Corcym srl. CD is THV proctor for Edwards Lifesciences. SJ receives speaker fees from Abiomed. PV is consultant to Corcym srl and he receives travel support from Corcym srl. BM is consultant to Corcym srl. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures described in this article were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying videos. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kostyunin AE, Yuzhalin AE, Rezvova MA, et al. Degeneration of Bioprosthetic Heart Valves: Update 2020. J Am Heart Assoc 2020;9:e018506. [Crossref] [PubMed]
- Capodanno D, Petronio AS, Prendergast B, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017;38:3382-90. [Crossref] [PubMed]
- Tamagnini G, Bourguignon T, Rega F, et al. Device profile of the Inspiris Resilia valve for aortic valve replacement: overview of its safety and efficacy. Expert Rev Med Devices 2021;18:239-44. [Crossref] [PubMed]
- Meuris B, De Praetere H, Strasly M, et al. A novel tissue treatment to reduce mineralization of bovine pericardial heart valves. J Thorac Cardiovasc Surg 2018;156:197-206. [Crossref] [PubMed]
- Lamberigts M, Szecel D, Rega F, et al. Sutureless aortic valves in isolated and combined procedures: Thirteen years of experience in 784 patients. J Thorac Cardiovasc Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- Szecel D, Lamberigts M, Rega F, et al. Avoiding oversizing in sutureless valves leads to lower transvalvular gradients and less permanent pacemaker implants postoperatively. Interact Cardiovasc Thorac Surg 2022;35:ivac157.
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Vanhaverbeke M, De Backer O, Dubois C. Practical Approach to Transcatheter Aortic Valve Implantation and Bioprosthetic Valve Fracture in a Failed Bioprosthetic Surgical Valve. J Interv Cardiol 2022;2022:9899235. [Crossref] [PubMed]
- Amabile N, Zannis K, Veugeois A, et al. Early outcome of degenerated self-expandable sutureless aortic prostheses treated with transcatheter valve implantation: A pilot series. J Thorac Cardiovasc Surg 2016;152:1635-7. [Crossref] [PubMed]
- Ribeiro HB, Rodés-Cabau J, Blanke P, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J 2018;39:687-95. [Crossref] [PubMed]
- Tarantini G, Nai Fovino L. Coronary Access and TAVR-in-TAVR: Don't Put Off Until Tomorrow What You Can Do Today. JACC Cardiovasc Interv 2020;13:2539-41. [Crossref] [PubMed]
Cite this article as: Dubois C, Minten L, Lamberigts M, Lesizza P, Jacobs S, Adriaenssens T, Verbrugghe P, Meuris B. Valve-in-valve transcatheter aortic valve replacement for the degenerated rapid deployment PercevalTM prosthesis: technical considerations. J Vis Surg 2023;9:46.


