
Video-assisted thoracoscopic left S8 segmentectomy guided by pre-operative 3D reconstruction in a patient with synchronous bilateral primary lung cancer: a case report
Highlight box
Key findings
• A novel cloud-based 3D reconstruction platform can be used to aid performing complex thoracoscopic segmentectomy
What is known and what is new?
• Preoperative 3D reconstruction can help overcome challenges associated with performing thoracoscopic segmentectomy. Various software solutions are available, but it is not known if there one which is superior to the others. This report highlights a new 3D reconstruction software solution that may more accessible than the others due to the cloud-based nature of the platform.
What is the implication, and what should change now?
• Further study is required to determine if cloud-based platforms such as this one confer additional benefits over non-cloud-based platforms.
Introduction
Background
Lobectomy has long been regarded as the surgical operation of choice for early stage lung cancer (1), while sublobar resections were generally reserved for patients who could not tolerate lobectomy due to advanced age, insufficient pulmonary function or other significant co-morbidities (2). Moreover, in patients with synchronous bilateral primary lung cancer, contralateral sublobar resections have been widely used with excellent long-term outcomes (3,4).
However, the recent JCOG0802 and CALGB140503 trials have shown that sublobar resections particularly segmentectomy, may be equivalent if not superior to lobectomy for otherwise healthy patients with peripheral lung cancers smaller than 2 cm in size, with no signs of lymph node or distant metastases (5,6). However, segmentectomy remains a more difficult operation than lobectomy (7), even more so for complex segmentectomies involving resection of more than one inter-segmental plane such as the individual segments of the upper lobes or basilar segments of the lower lobes compared to simple segmentectomies involving resection of one intersegmental plane such as a lingulectomy or superior segmentectomy of the lower lobes (8).
Rationale and knowledge gap
As a result of the technical challenges of performing a segmentectomy, 3D reconstructions of a patient’s computed tomography (CT) images can be used to improve surgical planning and may result in better surgical outcomes including decreased operative times, intra-operative blood loss and incidence of post-operative air leaks (9). Furthermore, the use of 3D reconstruction enhanced with virtual reality visualization may also significantly improve pre-operative planning for segmentectomy (10). Future prospective studies, such as the DRIVATS trial, are being performed to confirm the value of routine use of 3D reconstruction for lung resections, particularly for segmentectomy given the inherent additional complexity of segmentectomy over lobectomy (11). To this end, various software solutions have been described for planning lung resections including PulmoVR (10), Fujifilm Synapse 3D (12), Materialise Mimics (13), OsiriX (14) 3D Slicer (15) and Visible Patient (16) but it is uncertain if any one is superior to another. Moreover, these generally require a dedicated console or viewing device to access and manipulate the 3D reconstruction. A cloud-based platform that is more easily accessible to end-users may offer benefits over the various software solutions currently available.
Objective
I present this case of a complex segmentectomy (left S8 segmentectomy) in a patient with synchronous bilateral primary lung cancer which was facilitated by pre-operative 3D reconstruction for surgical planning done with by a novel cloud-based medical imaging 3D reconstruction service to demonstrate its utility, in accordance with the CARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-19/rc).
Case presentation
A 62-year-old male ex-smoker with a 30 pack-year history of smoking and a background of hypertension, hyperlipidemia, benign prostatic hyperplasia as well as a previous coronary artery bypass grafting (CABG) done in October 2020 for triple vessel coronary artery disease, was seen by us for the problem of a newly diagnosed right lower lung adenocarcinoma on the background of bilateral lower lobe nodules in September 2022. The patient was asymptomatic from the lung nodules and they were incidentally detected on a CT thorax done as part of his work-up for his CABG in October 2020. These nodules showed gradual increase in size, so a CT guided biopsy of the largest nodule in the right lower lobe was performed in September 2022 which demonstrated adenocarcinoma.
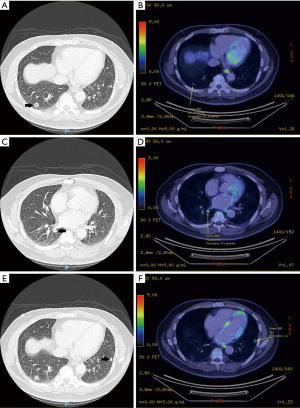
The staging positron emission tomography (PET) scan done for the patient in September 2022 revealed that in addition to the biopsy-proven lesion in the lateral segment of the right lower lobe which measured 1.2 cm and had a maximum standardized uptake value (SUVmax) of 1.1 (Figure 1A,1B), there was another 1.2 cm solid nodule in the superior segment of the same lobe with SUVmax 4.1 in the same lobe (Figure 1C,1D), as well as a separate 0.9 cm solid nodule in the anterior segment of the left lower lobe with SUVmax 3.6 (Figure 1E,1F). Otherwise, there was no evidence of hypermetabolic lymphadenopathy or other distant metastases on the PET. Magnetic resonance imaging of the brain also showed no metastases in October 2022. Lung function testing demonstrated a forced expiratory volume in 1 second of 2.21 L (74% predicted) and diffusion capacity of the lung for carbon monoxide of 8.37 mM/min/kPa (95% predicted). The patient had a maximum oxygen consumption of 15.8 mL/kg/min on cardiopulmonary exercise testing.
This case was discussed at a multi-disciplinary tumor board which concluded the patient likely had synchronous bilateral early-stage lung cancer based on the absence of nodal or distant metastases on staging investigations as well as the slow rate of growth of all three lesions and thus the recommendation was for surgical resection. Endobronchial ultrasound was not offered by our pulmonologists in this case as there were no enlarged or hypermetabolic mediastinal lymph nodes seen on the PET. A two staged operation was planned, with a left S8 segmentectomy as the first operation to remove the left sided lesion and preserve sufficient lung function in order to allow for one lung ventilation for the second operation, which would be a right lower lobectomy to remove both right sided lesions. For the left sided lesion, we chose to perform a left S8 segmentectomy instead of a wedge resection as segmentectomy may have superior oncological outcomes compared to a wedge resection if adequate margins can be achieved, and this was confirmed on pre-operative 3D reconstruction as discussed below. For the right sided lesions, a sublobar resection with adequate margins would have been difficult to perform the lesion in the superior segment as it was quite central and close to the inferior pulmonary vein. Moreover, although we suspected pre-operatively that they were two separate primaries in the right lung (i.e., two T1 lesions in the right lower lobe) based on their slow growth, we were also concerned of the possibility that one of them may have been an intra-lobar metastasis of the other (i.e., a T3 lesion in the right lower lobe), and thus the recommendation of our multi-disciplinary tumor board was for a lobectomy for the two right lower lobe lesions.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript as well as the accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Pre-operative preparation (Figures 2,3)
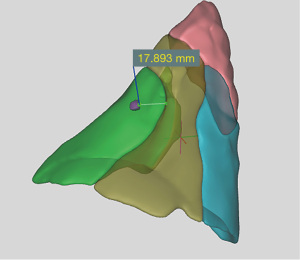
A 3D reconstruction of the patient’s CT thorax images was performed using a novel cloud-based medical imaging 3D modeling service, Hexa3D (HexaLotus Technology Pte Ltd, Singapore, Singapore) for pre-operative planning for the left S8 segmentectomy. Anonymized images were retrieved from our Radiology department and sent to Hexa3D for processing. After an average of 1–2 working days, a fully interactive 3D reconstruction of the patient’s lungs (complete with anatomical details of bronchovascular structures, segmental boundaries and the lesion of concern) was made available to us on a secure web link that was accessible on any internet-enabled device. Of note, the software creates segmental boundaries based on the arterial blood supply of the segment of interest.
Based on the 3D reconstruction, the lesion of concern was localized to the left S8 segment and an estimated minimum margin of resection of 1.8 cm would be obtained with an isolated left S8 segmentectomy (Figure 2). This estimated margin of resection could not have been measured on 2D CT images, and is an important pre-requisite for determining whether a sublobar resection would be appropriate and also whether a segmentectomy or wedge resection could give a better margin of resection.
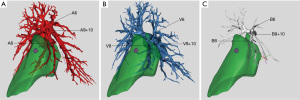
The precise anatomical details (including anatomical variations and the relative positions) of the bronchovascular structures that needed to be resected for the planned segmentectomy were also carefully studied prior to surgery (Figure 3A-3C). The 3D reconstruction demonstrated that the patient’s segmental anatomy displayed a fairly classical branching pattern, with a separate A8 artery and common A9+10 arterial trunk and as well as a separate B8 bronchus and common bronchial B9+10 trunk (17). Although there was a common V9+10 venous trunk, there were two separate V8 vein branches draining the S8 segment that would have to be divided during the segmentectomy. While study of the 2D CT images would allow us to do this as well, this is made much easier with the 3D reconstruction and allows us to resect the two separate V8 vein branches confidently during surgery without fearing that either one may be draining any of the adjacent segments.
Procedure (Video 1)
The operation was performed in November 2022. The patient underwent general anesthesia using a double lumen endotracheal tube for single lung ventilation. He was positioned in the right lateral position and had a paravertebral block using 20 mL ropivacaine 0.5% administered by the anesthetist under ultrasound guidance. A 3-port video-assisted thoracoscopic surgery (VATS) approach was then used for this case. A 3 cm utility port incision was made the 4th intercostal space at the anterior axillary line, followed by a 1 cm camera port incision at the 7th intercostal space mid-axillary line and another 1 cm posterior port incision just below the tip of the scapula. An “X-Small” sized Alexis wound protector (Applied Medical, Rancho Santa Margarita, California) was used at the utility port, and a 10 mm, 30-degree video telescope (Karl Storz, Tuttlingen, Germany) was introduced to inspect the thoracic cavity thoracoscopically.
As the patient had a previous CABG, there were adhesions encountered within the thoracic cavity, so careful adhesiolysis was first performed to fully mobilize the lung using a combination of sharp and blunt dissection. Next, the interlobar artery was dissected out within the fissure using electrocautery and Ligasure (Medtronic Inc., Minneapolis, MN, USA) dissection. Pulmonary artery branches to the lower lobe were dissected out and all segmental branches were exposed. The A8 artery was identified, encircled with a thoracoscopic right angle and then transected with an EndoGIA stapler (Medtronic Inc., Minneapolis, MN, USA). The remaining A9+10 artery was dissected out, encircled and retracted away from the underlying bronchi using a silk ligature. Next, the lymph node between B8 and B9+10 bronchi was dissected out and sent for frozen section. The inferior pulmonary ligament was divided and the station 9 and 10 lymph nodes dissected out and sent for frozen section. Upon confirmation that there was no evidence of malignancy in these lymph nodes, we proceeded with the planned isolated S8 segmentectomy.
The B8 bronchus was dissected out and encircled with a thoracoscopic right angle. An inflation test was performed after the B8 bronchus was occluded with an EndoGIA stapler, to ensure that the remaining lower lobe segments could expand before the B8 bronchus was transected with the EndoGIA stapler. Next, the pulmonary veins were dissected out and the two V8 vein branches identified on pre-operative 3D reconstruction were encircled with a thoracoscopic right angle before they were transected with an EndoGIA stapler.
In order to help identify the intersegmental plane between S8 and S9+10, 0.5 mg/kg dose of intravenous indocyanine green (ICG) (Diagnostic Green GmbH, Aschheim-Dornach, Germany) was administered (18). After 10–20 seconds, a demarcation line appears between the lung parenchyma that was highlighted by ICG dye and the lung parenchyma that was not highlighted by ICG on near-infrared fluorescence thoracoscopy, allowing the demarcation line to be marked with electrocautery. This demarcation line was then transected with serial firing of an EndoGIA stapler to complete the segmentectomy. At the proximal part of the segmentectomy specimen, care was taken to maneuver the EndoGIA stapler in a manner that would ensure that the distal stumps of the previously transected bronchovascular structures were included in the segmentectomy specimen, while also avoiding the other bronchovascular structure leading to the remaining lower lobe.
Upon completion of the segmentectomy, the specimen was retrieved from the chest and the hilar anatomy was inspected to ensure that the remaining bronchovascular structures were preserved appropriately. Water was introduced into the chest cavity and an insufflation test was performed to examine for air leak and to ensure that the remaining lower lobe segments could re-expand. After that, a systematic lymph node dissection was performed and hemostasis secured as per routine prior to chest tube placement at the conclusion of the operation. Total operative time was 230 minutes and estimated blood loss was less than 50 mL.
Post-operative course (Figure 4)
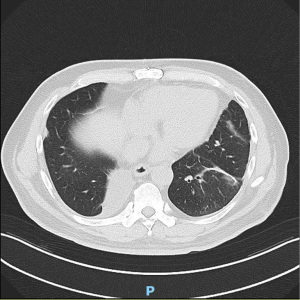
The patient was extubated after surgery in the operating room and had an uneventful post-operative course. His chest tube was removed on post-operative day 4 and he was discharged the day after. Histopathological analysis revealed a completely resected 1.2 cm adenocarcinoma (complex glandular subtype) with a 1.5 cm resection margin and 8 negative lymph nodes from stations 7, 8, 9, 10, 11, 12 and 13.
As patient recovered well, he subsequently underwent a right VATS lower lobectomy a month later in December 2022, which demonstrated a complete resection of two separate foci of adenocarcinoma (one 1.5 cm papillary subtype adenocarcinoma, and a separate 1.1 cm complex glandular subtype adenocarcinoma) and 34 negative lymph nodes from stations 4R, 7, 8, 9, 10, 11, 12 and 13. Molecular profiling of each lesion was performed and they were determined to be three separate T1N0M0 adenocarcinomas, each with a distinct molecular profile, so no further adjuvant treatment was administered. The patient remains well and the latest post-operative CT thorax done in August 2023 showed good re-expansion of all remaining lung parenchyma with no evidence of recurrence so far (Figure 4).
Discussion
Segmentectomy remains a more difficult operation than lobectomy with several key challenges. Firstly, it requires meticulous patient selection for best oncological outcomes. The lesion of concern should be located in the peripheral one third of the lung (5,6) and the planned segmentectomy should achieve a margin of resection to size of tumor ratio of at least 1 (19,20). Secondly, the operating surgeon must be familiar with the various branching patterns and anatomical variations of the segmental bronchovascular structures to allow for a safe and accurate segmentectomy that avoids compromise of the adjacent segments (21). Thirdly, the hilar dissection of bronchovascular structures is more difficult in a segmentectomy, as it necessitates the surgeon to dissect more distally than in a lobectomy (22). Finally, identifying the intersegmental plane intra-operatively is often cited as the most difficult part of performing a segmentectomy, as the intersegmental plane is usually not evident intra-operatively, unlike the oblique and horizontal fissures which help demarcate the plane between the lobes of the lung while performing a lobectomy (23).
To this end, pre-operative 3D reconstruction aids the surgeon in several important ways in planning for curative resection of a peripherally located lung cancer and helps overcome many of the technical challenges described above:
- Firstly, it helps determine whether a lobectomy or sublobar resection should be performed, based on a particular lesion’s size, location and whether an adequate margin of resection is achievable. A lobectomy may be more suitable if a lesion is not located in the outer third of the lung and a margin of resection of at least the size of the lesion cannot be obtained.
- Secondly, if a sublobar resection is being planned, it assists in deciding whether an anatomical segmentectomy or non-anatomical wedge resection would provide a better margin resection. In cases when a planned segmentectomy cannot achieve a margin of resection of at least the size of the lesion, it may be more suitable to either include the adjacent segment(s) in the surgical plan or to perform a non-anatomical wedge resection instead.
- Thirdly, if a segmentectomy is being planned, it guides the surgeon in choosing the particular segments that needs to be resected for an adequate margin of resection. As mentioned above, if a planned segmentectomy is unable to achieve an adequate margin of resection, additional segments may need to be included in the surgical plan to ensure oncological efficacy.
- Next, if a segmentectomy is being planned, it will provide precise anatomical details (including anatomical variations and the relative positions) of the bronchovascular structures that need to be resected for the planned segmentectomy. This allows the surgeon to have a mental model of what structures need to ligated or preserved as well as where they lie in relation to each other during the segmentectomy, thus facilitating the hilar dissection of the bronchovascular structures.
- Finally, it also provides the surgeon with a virtual roadmap of the inter-segmental plane(s) that needs to be divided during the planned segmentectomy. In cases of a complex segmentectomy, the use of an adjunct technique like negative staining with intravenous ICG after division of segmental arterial branches (18) such as was used in this case also helps identify the inter-segmental planes that need to be divided during surgery.
We described the technique which we used for a complex segmentectomy (left VATS S8 segmentectomy) in a patient whose surgery was made more challenging because of his previous CABG but assisted by pre-operative 3D reconstruction. In addition to the benefits of pre-operative 3D reconstruction described above, we would also like to highlight several technical tips that can be seen in the video accompanying this manuscript:
- In patients with a previous CABG, the densest adhesions of the left upper lobe are usually to the chest wall, and these require more sharp dissection with appropriate retraction of the lung away from the chest wall. However, softer adhesions form between the left upper lobe and the heart and pericardium, likely because the constant movement of the heart prevents the lung from adhering too much to the heart and pericardium. These softer adhesions are amenable to blunt dissection to create a tunnel between the hilum of the left upper lobe to the aorto-pulmonary window area. This tunnel facilitates retraction of the remaining left upper lobe for sharp dissection of the denser adhesions anteriorly, or to fire a stapler from this tunnel to free most of the left upper lobe and leave a narrow strip of lung tissue stuck to the anterior chest wall in cases where the adhesions to the chest wall are too dense and there is concern that injury to an underlying left internal mammary artery bypass graft.
- It is useful to dissect out all segmental arteries in the interlobar fissure leading to the left lower lobe, to facilitate identification of the correct artery leading to the segment of interest as seen on the 3D reconstruction. The position of the segmental artery of interest and its relationship with adjacent arteries should correspond to what is seen on the 3D reconstruction before it is divided.
- Placing a silk ligature around the adjacent segmental artery (e.g., A9+10 in this case) allows us to retract it away to facilitate dissection of the underlying lymph node and bronchus leading to S8. This is an important trick that can be used many other segmentectomies, as there is often an overlying vein or artery leading to an adjacent segment that needs to be preserved but yet obstructs adequate exposure of a critical underlying bronchovascular structure that needs to be divided for a segmentectomy.
- After injection of intravenous ICG and marking the inter-segmental border of interest, it can be compared against predicted inter-segmental border seen on the 3D reconstruction to ensure an accurate parenchymal resection is performed to complete the segmentectomy. This is an important step to ensure that the margin of resection obtained from the segmentectomy would closely match what is predicted on the 3D reconstruction, particularly for non-palpable lesions.
In contrast to other currently available 3D reconstruction software solutions (10-16) available for lung resections, the service which we have used in this case allows the 3D reconstruction to be accessible anywhere via any internet-enabled device with a web browser (e.g., in the operating room, clinic or multi-disciplinary tumor board) given the cloud-based nature of the platform which is a novel feature of this service. Although this software does not have United States Food and Drug Administration (FDA) or European Union CE clearance, it does have local clearance for use in Singapore and China and is used in a number of hospitals in those countries. We have found it to be highly accurate with the segmental boundaries closely corresponding to our intra-operative technique of identifying the inter-segmental plane (i.e., negative staining with intravenous ICG after division of segmental arterial branches) and it is of great help during surgery. In particular, the ability to interact with the 3D reconstruction in the operating room via a laptop connected to the Internet allows us to have a constant visual guide of the relevant bronchovascular structure we are intending to resect and we can continuously refer back to it while the relevant structures is being dissected out intra-operatively. Transection of the structure is only performed once we are satisfied that the bronchovascular structure in question matches what is seen on the reconstruction. Whenever interaction with the 3D reconstruction is required, an unscrubbed member of the team instructed by the surgeon would manipulate the 3D reconstruction on the laptop. This is done quite smoothly as the software has an easy and simple to use interface.
However, this case report’s main limitation is that it only highlights a single patient in which we have successfully used this novel cloud-based 3D reconstruction software. While this report demonstrates the feasibility of use for this platform in pre-operative planning for complex thoracoscopic segmentectomy, it is difficult to draw further conclusions over its potential benefits over non cloud-based platforms. Further studies comparing cloud-based platforms such as this are also necessary to determine if they confer additional benefits over non-cloud-based platforms. Regardless, it is clear that there will be a greater need for these 3D reconstructions in the era of more widespread application of segmentectomy.
Conclusions
Segmentectomy is an excellent surgical option for patients with synchronous bilateral primary lung cancers and small peripheral lung cancers, although it is a more technically challenging operation than a lobectomy. Use of this novel cloud-based 3D reconstruction software is feasible for planning complex thoracoscopic segmentectomy, but further work is required to determine if such cloud-based platforms confer additional benefits over non-cloud-based platforms. Nonetheless, as the indications and adoption of segmentectomy continue to grow, we will likely see an increase in the use of pre-operative 3D reconstructions (either cloud-based or non-cloud-based) to facilitate these operations and its routine use may become the standard of care in the future.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the CARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-19/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-19/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-19/coif). BHO reports receiving personal fees from AstraZeneca, Bristol-Myers Squibb, Roche, Medtronic and MSD and travel support from MSD outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript as well as the accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Wang C, Wu S, Zhang R, et al. Identifying Lung Cancer Patients Suitable for Segmentectomy: A Brief Review. Front Surg 2021;8:637441. [Crossref] [PubMed]
- Yang H, Sun Y, Yao F, et al. Surgical Therapy for Bilateral Multiple Primary Lung Cancer. Ann Thorac Surg 2016;101:1145-52. [Crossref] [PubMed]
- Watanabe T, Tanahashi M, Suzuki E, et al. Surgical treatment for synchronous multiple primary lung cancer: Is it possible to achieve both curability and preservation of the pulmonary function? Thorac Cancer 2021;12:2996-3004. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Yang CJ, Fitch ZW, Balderson SS, et al. Anatomic thoracoscopic segmentectomy for early-stage lung cancer. J Vis Surg 2017;3:123. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Vervoorn MT, Wulfse M, Mohamed Hoesein FAA, et al. Application of three-dimensional computed tomography imaging and reconstructive techniques in lung surgery: A mini-review. Front Surg 2022;9:1079857. [Crossref] [PubMed]
- Bakhuis W, Sadeghi AH, Moes I, et al. Essential Surgical Plan Modifications After Virtual Reality Planning in 50 Consecutive Segmentectomies. Ann Thorac Surg 2023;115:1247-55. [Crossref] [PubMed]
- Niu Z, Chen K, Jin R, et al. Three-dimensional computed tomography reconstruction in video-assisted thoracoscopic segmentectomy (DRIVATS): A prospective, multicenter randomized controlled trial. Front Surg 2022;9:941582. [Crossref] [PubMed]
- Rojas D, Lenot B, Pfeuty K. Throacoscopic subxiphoid right S9+10 segmentectomy: a case report. J Vis Surg 2023;9:19. [Crossref]
- Wu YJ, Shi QT, Zhang Y, et al. Thoracoscopic segmentectomy and lobectomy assisted by three-dimensional computed-tomography bronchography and angiography for the treatment of primary lung cancer. World J Clin Cases 2021;9:10494-506. [Crossref] [PubMed]
- Yao F, Wang J, Yao J, et al. Three-dimensional image reconstruction with free open-source OsiriX software in video-assisted thoracoscopic lobectomy and segmentectomy. Int J Surg 2017;39:16-22. [Crossref] [PubMed]
- Xue L, Fan H, Shi W, et al. Preoperative 3-dimensional computed tomography lung simulation before video-assisted thoracoscopic anatomic segmentectomy for ground glass opacity in lung. J Thorac Dis 2018;10:6598-605. [Crossref] [PubMed]
- Le Moal J, Peillon C, Dacher JN, et al. Three-dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: a pilot study. J Thorac Dis 2018;10:196-201. [Crossref] [PubMed]
- Nomori H, Okada M. Illustrated anatomical segmentectomy for lung cancer. Springer, 2012:193.
- Ng CS, Ong BH, Chao YK, et al. Use of Indocyanine Green Fluorescence Imaging in Thoracic and Esophageal Surgery. Ann Thorac Surg 2023;115:1068-76. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 932-3. [Crossref] [PubMed]
- Liu W, Lai H, Wang Z, et al. Does surgical margin affect recurrence and survival after sublobar pulmonary resection for lung cancer? Interact Cardiovasc Thorac Surg 2022;34:1089-94. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2017;65:343-9. [Crossref] [PubMed]
- Mendogni P, Tosi D, Rosso L, et al. VATS segmentectomy: an underused option? J Vis Surg 2017;3:136. [Crossref] [PubMed]
- Meacci E, Nachira D, Congedo MT, et al. Uniportal video-assisted thoracic lung segmentectomy with near infrared/indocyanine green intersegmental plane identification. J Vis Surg 2018;4:17. [Crossref] [PubMed]
Cite this article as: Ong BH. Video-assisted thoracoscopic left S8 segmentectomy guided by pre-operative 3D reconstruction in a patient with synchronous bilateral primary lung cancer: a case report. J Vis Surg 2023;9:45.


