The future of sutureless valve technology
Introduction
Background
Surgical aortic valve replacement (SAVR) has been established as the gold standard treatment modality for patients with severe symptomatic aortic stenosis (1). However, the consistent increase in the adoption of minimally invasive techniques fomented the need for a reliable valvular prosthesis that can facilitate these techniques and improve surgical efficiency without compromising clinical outcomes. The widespread use of the sutureless valves (SV) technology has been fueled by their relatively straightforward and fast deployment technique and the encouraging mid- and long-term outcomes compared to conventional SAVR. The PercevalTM aortic valve bioprosthesis (LivaNova Group, Milan, Italy) is a bovine pericardial sutureless valvular prosthesis with a self-expanding nitinol alloy stent.
Rationale and knowledge gap
Clinical studies have demonstrated that this valve can provide excellent hemodynamic performance compared to the stented bioprosthesis (2). However, there have always been concerns regarding their utility in particular clinical situations, such as patients with bicuspid aortic valves (BAV) and/or patients with dilated aortic annuli. Also, this technology has historically been associated with increased rates of paravalvular leak (PVL) and a higher need for permanent pacemaker implantation (PPI) (2,3).
Objective
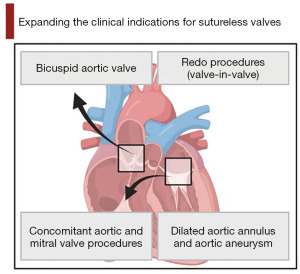
In this review, we explore the future of SV technology with particular emphasis on the potential device improvements and the expanding indications for the use of this technology (Figures 1,2).
New advances and clinical applications of the SV technology
The new FREE tissue treatment technology
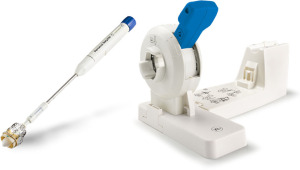
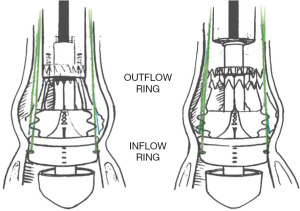
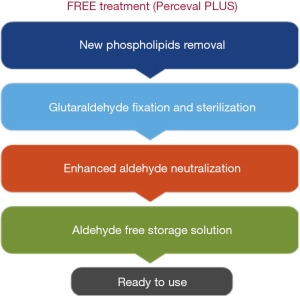
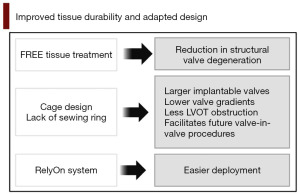
The introduction of the new generation PercevalTM PLUS valve (Figure 3) and the accompanying RelyOn Deployment System (Figures 4,5) represents an important improvement in the design of this valvular prosthesis. In addition to improving the deployment technique, the innovative FREE tissue treatment (Figure 6), which incorporates a phospholipid reduction and aldehyde neutralization process, can potentially reduce structural valve degeneration and improve long-term surgical outcomes (4,5). In comparison with other commercially available technologies, such as the Linx- and ThermaFix-treated valvular prosthesis, the FREE-treated pericardial leaflets showed the lowest levels of residual aldehyde molecules and comparable levels of phospholipid and calcification burdens (5).
There are currently no completed prospective clinical trials on the outcomes of the new PercevalTM PLUS valve in humans. However, in their retrospective study, Lamberigts and colleagues reviewed the outcomes of 784 PercevalTM patients versus 146 PercevalTM PLUS patients who underwent aortic valve replacement (AVR) with or without combined procedures between 2007 and 2021. Their results showed no significant difference between the two groups in terms of major adverse events, in-hospital mortality, or the need for dialysis. Interestingly, PPI rates were significantly lower in the PercevalTM PLUS XL size group (12.9% vs. 3.1%, P<0.001), which was attributed by the authors to the low inflow height of this prosthesis. The PercevalTM PLUS valve also showed significant improvement in trans-valvular gradients (peak gradient: 26±10 vs. 21±8 mmHg, P<0.001, mean gradient: 14±5 vs. 12±5 mmHg, P<0.001); however, the authors explained that this improvement might be due to increased experience with the use of the valve (4).
Reducing the risk of PPI after SV
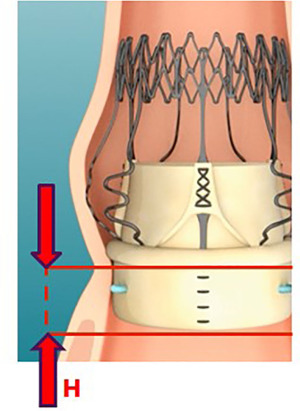
While the development of conduction abnormalities that may require PPI is likely a multifactorial process, it has been observed that the use of SVs was associated with higher rates of PPI than SAVR using a stented bioprosthesis. A minimal radial force on the aortic wall is necessary for stabilizing the SVs within the aortic root, which may compromise the bundle of His. However, the rates of PPI with SVs have been improving in recent years, with 30-day PPI rates following SVs ranging from 3.3–4.4% in recent studies (6-8). This may be partly due to more surgeon exposure and experience in using these valves, a better understanding of the risk factors for PPI, and the improvement in the sizing techniques of these valves (2,9,10). Lorusso and colleagues demonstrated that increased rates of early PPI in the PercevalTM sutureless AVR (Su-AVR) cohort correlated with the XL valve size and with patients who had preoperative conduction disorders (11). Other risk factors for PPI following AVR included extensive annular debridement (2), BAV, female sex, older age, prolonged cardiopulmonary bypass times, and concomitant procedures (12).
The new generation PercevalTM PLUS XL valve may offer a solution to this problem with its adapted design that reduces the protrusion of the valve into the left ventricular outflow tract (LVOT), which may decrease the risk of damage to the conduction system (11). In addition, preoperative multidetector-row cardiac computed tomography (MDCT) has been recently proven useful in determining the aortic root dimensions and optimizing the sizing of the valve prosthesis (13). Margaryan and colleagues demonstrated in a study of 54 patients that MDCT-derived estimates of prosthesis size may have higher predictive values when compared to echocardiographic measurements, concluding that MDCT may be a valuable tool for precise aortic annulus measurements and selecting the optimal size valve prosthesis (14).
Reducing the risk of PVL after SV
Similarly, the incidence of PVL is one of the main concerns and a persistent finding after the use of SVs, especially in patients with BAV and dilated aortic annulus (15,16). Limited studies have recognized specific risk factors for developing PVL; however, those reported include malpositioning of the valve within the aortic annulus, incomplete expansion of an improperly sized valve, or incomplete aortic annulus decalcification (3,6,17). In their study, Erfe and colleagues reported comparable PVL rates between SVs and traditional stented bioprosthetic valves, which may also be partly due to the experience of the surgical team and the standardization of the implantation process of the SVs (12). Similarly, Shrestha and colleagues reported early and late mortality and complication rates, including PVL, to be comparable to published reports for traditional AVR (9). However, in their systematic review and meta-analysis, Jolliffe and colleagues reported a rate of 3.6% PVL compared to conventional stented bioprosthetic valves (less than 1%) (3). Proper sizing of SV has garnered attention in recent years, as determining the correct valve size is critical. This not only helps lower the occurrence of PVL but also prevents the incidence of incomplete expansion and leaflet opening or stent frame infolding that can arise from under-sizing the valve (18). The introduction of preoperative MDCT sizing protocols in the future may be helpful in optimal valve size selection and improving the rates of PVL; however, this needs to be proven by data derived from wide-scale clinical studies.
The use of SV in patients with BAV
The use of SVs in BAV replacements has been limited by anatomical challenges presented by the elliptic aortic annulus shape of a bicuspid valve, resulting in concerns of increased rates of PVL (15). Recent studies have demonstrated that SV may be a viable option for these patients. In a recent review, our group reported that using SVs may offer comparable implantation success rates in patients with BAV compared to those with tricuspid valves. Also, the reported mean post-operative trans-valvular gradients and mean postoperative aortic valvular areas were comparable across all studies. However, this was at the expense of higher complication rates, including new-onset atrial fibrillation, PVL, new onset atrioventricular block, and pacemaker insertion (19). These findings suggest that using SVs in the context of BAV may be feasible when performed by experienced teams. Similarly, in another review, Vendramin and colleagues reported that the PercevalTM valve can be safely deployed in patients who present with a BAV, without additional risk of PVL. They concluded that while an AVR with SVs is a technically more demanding procedure, the presence of a BAV should not be a contraindication for the use of this type of valve (20).
The use of SV in dilated aortic annulus
The use of SV to manage aneurysmal dilation of the aortic root has traditionally been associated with concerns regarding its ability to anchor securely to the aortic annulus (16). However, in their 2018 study, Lio and colleagues described a surgical approach for deploying the PercevalTM valve for concomitant AVR and ascending aortic replacement through an upper mini-sternotomy approach. The authors highlighted the importance of two anatomical sites in establishing a stable positioning of the prosthesis: the native aortic annulus and the sinotubular junction (STJ). Recreation of the STJ utilizing a Dacron graft allows for sufficient stability and decreases the opportunity for dislodgement. They also described the importance of performing the proximal anastomosis of the tubular graft prior to releasing the prosthesis for adequate sealing and to minimize the risk of manipulation of the SV device, thus reducing the risk of displacement. The study showed encouraging outcomes, with no reported PVL or prosthesis dislodgement in a sample size of seven patients, suggesting that using the PercevalTM valve may be feasible in this context (16). Similarly, Ali Hassan and colleagues reported the use of the PercevalTM valve in the presence of dilated aortic root and aortic aneurysm. The authors described the placement of a sub-commissural annuloplasty suture (under the commissure between the right coronary and non-coronary cusps) to reduce the size of the dilated aortic annulus, allowing for the implantation of a PercevalTM XL valve. The SV prosthesis was successfully implanted without migration or displacement, suggesting that this technique may be an option in this group of patients (21).
The use of SV for combined aortic and mitral valve surgery
The use of SV in the context of concomitant AVR and mitral valve replacement (MVR) has been associated with concern that the mitral valve prosthesis could potentially disrupt the three-dimensional configuration of the aortic root and the LVOT, as well as directly interfere with the aortic valve prosthesis (22). However, recent studies have shown that SVs may be a viable and safe option in double valve procedures (23,24). Szecel and colleagues conducted a long-term follow-up study that demonstrated no added risk of prosthetic dysfunction or PVL with PercevalTM valves when combined with prosthetic MVR compared to isolated AVR (24). Similarly, Baran and colleagues demonstrated that using PercevalTM valves is technically feasible and safe in multiple valve surgery (23). To address the technical challenges arising from the proximity of the aortic and mitral valve devices, it was proposed that the preoperative measurements of the aorto-mitral distance using transesophageal echocardiography may be helpful (22), along with proper positioning of the mitral valve prosthesis, to avoid obstruction of the LVOT by the struts of the biological mitral valve (23). In addition, the use of MDCT preoperative aortic root assessment may be a helpful tool in studying these patients and planning the surgical technique in the future.
The use of SV in redo valve-in-valve procedures
SV may play a role in valve-in-valve redo procedures for degenerated bioprosthesis. While not extensively studied, there are few reported instances of the PercevalTM valve being used in this clinical scenario. In their study, Dhanekula and colleagues (25) found that the unique cage design and the lack of a sewing ring of the SV allowed for implanting a larger valve, resulting in low gradients and allowing for the potential use of valve-in-valve transcatheter aortic valve implantation (TAVI) in the future. The authors noted that the learning curve associated with using SVs and proper sizing of the valve were important factors in determining good surgical results. The authors also highlighted the role of SVs in redo procedures following previous root surgery, which could allow for avoiding redo root procedures and coronary reimplantation. The use of PercevalTM valves in this small study was associated with encouraging surgical outcomes with low rates of postoperative complications (25). In a series of three case reports of patients who underwent a valve-in-valve procedure with SVs for severe aortic regurgitation following AVR, Stoker and colleagues similarly reported no perioperative complications or in-hospital deaths, concluding that SV is a feasible option in this group of patients (26). Vendramin and colleagues highlighted the interesting use of SVs in a review of complex and challenging redo procedures. They discussed the benefit of utilizing SVs in valve-in-valve redo operations owing to the difficulties that may be encountered in positioning the anchoring stitches or a traditional stented prosthesis of adequate size due to a severely calcified and constricted annulus. This review reported the effective use of SVs in this context, with encouraging short and long-term outcomes (20).
The use of SV in infectious endocarditis
Valve replacements in infectious endocarditis cases can be complex and challenging operations, often requiring prolonged cross-clamp and ischaemic times. The use of SVs presents an opportunity to shorten the aortic cross-clamp time and to provide excellent hemodynamic performance without compromising safety (27,28). In a recent case report, Smith and colleagues presented an interesting case study of prosthetic valve endocarditis that was surgically treated by performing a bio-Bentall procedure in which a large PercevalTM SV was inserted into a 30 mm Dacron Valsalva graft with excellent surgical outcome (29). The authors highlighted the limitations in determining valve size preoperatively with the SV because annular size can vary based on the bulkiness of and relative position of the proximal suture line into the LVOT.
Nguyen and colleagues presented a case series of three patients with prosthetic valve endocarditis who underwent a redo valve-in-valve replacement utilizing the PercevalTM prosthesis. The authors described a technique in which the diseased leaflets were removed while leaving the previous bioprosthetic valve stent in place, into which the PercevalTM valve was implanted as per the manufacturer’s instructions. This technique mandates that all patients have decent tissue quality at the aortic root and no root abscesses. While the patients described in this study did not have recurrent infectious endocarditis, this technique is limited by possibly leaving behind residual infected tissue by leaving the previous stent in situ. However, it is a simpler procedure with reduced ischemic time and less residual foreign material, such as sutures and pledgets (30).
The reported rates of early and mid-term infectious endocarditis following insertion of SVs (1.6%) are similar to that of SAVR (3). In their study, Di Bacco and colleagues described a case study of a patient with an infected PercevalTM valve. The authors described the application of the “x-movement” technique to remove the infected and damaged valve, a technique that is typically described to facilitate the removal of malpositioned PercevalTM valves. The lack of anchoring valve sutures and the weakened adhesions due to infection allowed for the relatively quick valve extraction, thus reducing the risk of fragmentation and septic embolization (31).
The outcomes of SV versus TAVI
Over the last decade, TAVI has emerged as a valuable treatment option for intermediate and high-risk patients with severe aortic stenosis. However, SVs have also been utilized in this patient population when a direct surgical approach is favoured. This offers some advantages in reducing ischemic times and facilitating the conduction of minimally invasive approaches. In a systematic review and meta-analysis comparing Su-AVR vs. TAVI, Shinn and colleagues found that Su-AVR demonstrated lower early mortality (2.5% vs. 5%, P=0.02) and PVL rates (OR, 0.18; 95% CI: 0.11 to 0.30; P<0.0001). The two cohorts demonstrated similar rates of postprocedural stroke (OR, 0.71; 95% CI: 0.24 to 2.08; P=0.53) and PPI (OR, 0.884; 95% CI: 0.364 to 2.18; P=0.7) (32). In another systematic review and meta-analysis comparing Su-AVR vs. TAVI, Wang and colleagues reported that although associated with increased rates of bleeding and lengths of hospital stay, high-risk patients undergoing SVs showed lower rates of PVL (OR =0.06; 95% CI: 0.03 to 0.12, P<0.01), no differences in perioperative mortality (OR =0.55; 95% CI: 0.29 to 1.06, P=0.07) and lower mortality rates at both 1-year (OR =2.40; 95% CI: 1.40 to 4.11, P<0.01) and 2-year (OR =4.62; 95% CI: 2.62 to 8.12, P<0.01) follow-up (33). More recently, Muneretto and colleagues compared the long-term outcomes within an intermediate-risk profile cohort of elderly patients with severe aortic stenosis who underwent either Su-AVR or TAVI. Their results showed a significant reduction in 30-day mortality (Su-AVR =1.7% vs. TAVI =5.5%; P=0.024), rates of PPI (SV =5.5% vs. TAVI =10.7%, P=0.032) and rates of grade II or higher PVL (SV =1.3% vs. TAVI =9.8%, P<0.001). At 60 months, the Su-AVR cohort had a lower all-cause death rate (16.1%±4.1%) compared to TAVI (28.9%±5.3%) (34). These results suggest that Su-AVR may be a viable option for intermediate- and high-risk surgical patients who may not be suitable for TAVI.
The use of SV in minimally invasive AVR
One area which SV shows considerable utility is in minimally invasive aortic valve replacement (MI-AVR) techniques. Studies have shown that MI-AVR using conventional valvular prosthesis is typically associated with longer cross-clamp and cardiopulmonary bypass times than full sternotomy SAVR (35). Therefore, the incorporation of SV technology in MI-AVR procedures can potentially simplify the valve implantation technique and shorten the ischaemic times without compromising surgical outcomes. Past studies have demonstrated that the use of PercevalTM SV in MI-AVR procedures is safe and has been demonstrated to have good hemodynamic results, postoperative outcomes and 1-year survival (36). More recent studies have similarly shown promising results. In their study, Erfe and colleagues showed that using SV resulted in shorter procedure times and smaller incisions, with similar outcomes to conventional stented bioprosthesis. In their sub-analysis of patients who underwent MI-AVR, the use of SV was associated with a significantly higher need for PPI as compared to SAVR (9.6% vs. 3.8%, P<0.001) (12). Similarly, Andreas and colleagues showed that the MI-AVR group had less incidence of acute renal injury and dialysis than the full sternotomy group and that anterior right thoracotomy access had lower rates of stroke when compared to a mini-sternotomy approach (37). Fischlein and colleagues reported that using PercevalTM SV significantly reduced cross-clamp and cardiopulmonary bypass times in full sternotomy and mini-sternotomy procedures. In addition, the PercevalTM cohort displayed a significant reduction in major cerebral and cardiovascular events, new-onset atrial fibrillation rates, and re-hospitalizations at 1-year follow-up; however, this was at the expense of higher rates of PPI compared to stented bioprosthesis (38). Miceli and colleagues presented a study comparing patients undergoing a right anterior mini-thoracotomy (RT) with an SV versus TAVI. The RT group showed reduced in-hospital mortality (P=0.25) and incidence rate of stroke (P=0.3). The TAVI cohort also demonstrated 37.8% mild PVL and 27% moderate PVL, while the RT group only had 2.7% mild PVL. One- and two-year survival rates were 91.6% vs. 78.6% and 91.6% vs. 66.2% in the RT group versus the TAVI group, respectively (39).
Conclusions
There has been a rise in the use of SVs in cardiac surgery over the last decade. As technology advances and clinical experience grows, this trend will likely continue to increase. Future research focused on improving tissue durability and developing valve design may be key in the expansion of the use of this technology. In addition, better preoperative aortic root evaluation, using MDCT, and optimal valve size selection may help reduce surgical complications such as PVL and PPI. There may also be a role for these valves in certain clinical scenarios, such as patients with BAV, dilated aortic annulus, redo procedures, and patients requiring multiple valvular interventions; however, this needs to be endorsed by data derived from large-scale clinical studies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Aleksander Dokollari, Basel Ramlawi, and Gianluigi Bisleri) for the series “Sutureless Valves” published in Journal of Visualized Surgery. The article has undergone external peer review.
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-27/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-27/coif). The series “Sutureless Valves” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118:e523-661. [PubMed]
- Meco M, Montisci A, Miceli A, Panisi P, Donatelli F, Cirri S, Ferrarini M, Lio A, Glauber M. Sutureless Perceval Aortic Valve Versus Conventional Stented Bioprostheses: Meta-Analysis of Postoperative and Midterm Results in Isolated Aortic Valve Replacement. J Am Heart Assoc 2018;7:e006091. [Crossref] [PubMed]
- Jolliffe J, Moten S, Tripathy A, Skillington P, Tatoulis J, Muneretto C, Di Bacco L, Galvao HBF, Goldblatt J. Perceval valve intermediate outcomes: a systematic review and meta-analysis at 5-year follow-up. J Cardiothorac Surg 2023;18:129. [Crossref] [PubMed]
- Lamberigts M, Langenaeken T, Rega F, et al. Perceval Plus Versus Perceval: Impact Of Changes In Tissue And Valve Design On Early Outcome And Pacemaker Rates [abstract]. In: Heart Valve Society annual meeting 2023. 2023 March 29 - April 1; Malaga, Spain. Abstract nr D1.
- Meuris B, De Praetere H, Strasly M, et al. A novel tissue treatment to reduce mineralization of bovine pericardial heart valves. J Thorac Cardiovasc Surg 2018;156:197-206. [Crossref] [PubMed]
- Concistrè G, Bianchi G, Margaryan R, et al. Ten-year experience with sutureless Perceval bioprosthesis: single-centre analysis in 1157 implants. J Cardiovasc Med (Hagerstown) 2023;24:506-13. [Crossref] [PubMed]
- Niinami H, Sawa Y, Shimokawa T, et al. 1-year outcomes of patients implanted with the Perceval sutureless valve: the Japanese post-marketing surveillance study. Heart Vessels 2023;38:949-56. [Crossref] [PubMed]
- Glauber M, Di Bacco L, Cuenca J, et al. Minimally Invasive Aortic Valve Replacement with Sutureless Valves: Results From an International Prospective Registry. Innovations (Phila) 2020;15:120-30. [Crossref] [PubMed]
- Shrestha M, Fischlein T, Meuris B, et al. European multicentre experience with the sutureless Perceval valve: clinical and haemodynamic outcomes up to 5 years in over 700 patients. Eur J Cardiothorac Surg 2016;49:234-41. [Crossref] [PubMed]
- Berretta P, Andreas M, Carrel TP, et al. Minimally invasive aortic valve replacement with sutureless and rapid deployment valves: a report from an international registry (Sutureless and Rapid Deployment International Registry)†. Eur J Cardiothorac Surg 2019;56:793-9. [Crossref] [PubMed]
- Lorusso R, Ravaux JM, Pollari F, et al. Pacemaker implantation after sutureless or stented valve: results from a controlled randomized trial. Eur J Cardiothorac Surg 2022;62:ezac164. [Crossref] [PubMed]
- Erfe JM, Malaisrie SC, Andrei AC, et al. Outcomes of Sutureless/Rapid Deployment Valves Compared to Traditional Bioprosthetic Aortic Valves. Available online:
10.1016/j.athoracsur.2020.07.034 10.1016/j.athoracsur.2020.07.034 - Giuseppe Cerillo A, Pellegrini G, Laganà A, et al. Ct Based Sizing For Sutureless Aortic Valve Replacement [abstract]. In: Heart Valve Society annual meeting 2023. 2023 March 29 - April 1; Malaga, Spain. Abstract nr D15.
- Margaryan R, Kallushi E, Gilmanov D, et al. Sutureless Aortic Valve Prosthesis Sizing: Estimation and Prediction Using Multidetector-Row Computed Tomography. Innovations (Phila) 2015;10:230-5; discussion 235. [Crossref] [PubMed]
- Di Eusanio M, Berretta P. The sutureless and rapid-deployment aortic valve replacement international registry: lessons learned from more than 4,500 patients. Ann Cardiothorac Surg 2020;9:289-97. [Crossref] [PubMed]
- Lio A, Ferrarini M, Miceli A, et al. Sutureless Prosthesis Implantation and Ascending Aorta Replacement Through a Ministernotomy Approach [Internet]. 2018. Available online: www.innovjournal.com
- Mazine A, Teoh K, Bouhout I, et al. Sutureless aortic valve replacement: a Canadian multicentre study. Can J Cardiol 2015;31:63-8. [Crossref] [PubMed]
- Flynn CD, Williams ML, Chakos A, et al. Sutureless valve and rapid deployment valves: a systematic review and meta-analysis of comparative studies. Ann Cardiothorac Surg 2020;9:364-74. [Crossref] [PubMed]
- King M, Stambulic T, Payne D, et al. The use of sutureless and rapid-deployment aortic valve prosthesis in patients with bicuspid aortic valve: A focused review. J Card Surg 2022;37:3355-62. [Crossref] [PubMed]
- Vendramin I, Lechiancole A, Piani D, et al. Use of Sutureless and Rapid Deployment Prostheses in Challenging Reoperations. J Cardiovasc Dev Dis 2021;8:74. [Crossref] [PubMed]
- Ali Hassan SM, Paleczny S, Hamilton A, et al. Is It Feasible to Implant Sutureless Valves in Presence of Diseased Aortic Root and Aortic Aneurysm? Ann Thorac Surg 2022;113:e175-8. [Crossref] [PubMed]
- Minh TH, Mazine A, Bouhout I, et al. Expanding the indication for sutureless aortic valve replacement to patients with mitral disease. J Thorac Cardiovasc Surg 2014;148:1354-9. [Crossref] [PubMed]
- Baran C, Durdu MS, Gumus F, et al. Sutureless aortic valve replacement with concomitant valvular surgery. J Thorac Cardiovasc Surg 2018;155:2414-22. [Crossref] [PubMed]
- Szecel D, Eurlings R, Rega F, et al. Perceval Sutureless Aortic Valve Implantation: Midterm Outcomes. Ann Thorac Surg 2021;111:1331-7. [Crossref] [PubMed]
- Dhanekula AS, Nishath T, Aldea GS, et al. Use of a sutureless aortic valve in reoperative aortic valve replacement. JTCVS Tech 2022;13:31-9. [Crossref] [PubMed]
- Stoker T, Mashhour A, Easo J, et al. Novel Treatment of a Degenerated Bioroot With the Use of the Sutureless Valve Technique. Ann Thorac Surg 2018;105:e213-4. [Crossref] [PubMed]
- Lio A, Miceli A, Solinas M, et al. Initial Experience with Sutureless Sorin Perceval S Aortic Prosthesis for the Treatment of Prosthetic Valve Endocarditis. Thorac Cardiovasc Surg 2015;63:501-3. [Crossref] [PubMed]
- Glauber M, Kent WDT, Asimakopoulos G, et al. Sutureless Valve in Repeated Aortic Valve Replacement: Results from an International Prospective Registry. Innovations (Phila) 2021;16:273-9. [Crossref] [PubMed]
- Smith HN, Fatehi Hassanabad A, Kent WDT. Novel Use of the Perceval Valve for Prosthetic Aortic Valve Endocarditis Requiring Root Replacement. Innovations (Phila) 2022;17:67-9. [Crossref] [PubMed]
- Nguyen Q, White A, Lam W, et al. Valve-in-Valve Using Perceval Prostheses for Prosthetic Valve Endocarditis. Ann Thorac Surg 2022;114:e437-9. [Crossref] [PubMed]
- Di Bacco L, Pfeiffer S, Fischlein TJM, et al. Rapid Explantation of Rapid-Deployment Sutureless Valve in Case of Acute Endocarditis How to Remove Safely Sutureless Perceval S Prostheses. 2017. Available online: www.innovjournal.com
- Shinn SH, Altarabsheh SE, Deo SV, et al. A Systemic Review and Meta-Analysis of Sutureless Aortic Valve Replacement Versus Transcatheter Aortic Valve Implantation. Ann Thorac Surg 2018;106:924-9. [Crossref] [PubMed]
- Wang N, Tsai YC, Niles N, et al. Transcatheter aortic valve implantation (TAVI) versus sutureless aortic valve replacement (SUAVR) for aortic stenosis: a systematic review and meta-analysis of matched studies. J Thorac Dis 2016;8:3283-93. [Crossref] [PubMed]
- Muneretto C, Solinas M, Folliguet T, et al. Sutureless versus transcatheter aortic valves in elderly patients with aortic stenosis at intermediate risk: A multi-institutional study. J Thorac Cardiovasc Surg 2022;163:925-35.e5. [Crossref] [PubMed]
- Jahangiri M, Hussain A, Akowuah E. Minimally invasive surgical aortic valve replacement. Heart 2019;105:s10-5. [Crossref] [PubMed]
- Miceli A, Santarpino G, Pfeiffer S, et al. Minimally invasive aortic valve replacement with Perceval S sutureless valve: early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg 2014;148:2838-43. [Crossref] [PubMed]
- Andreas M, Berretta P, Solinas M, et al. Minimally invasive access type related to outcomes of sutureless and rapid deployment valves. Eur J Cardiothorac Surg 2020;58:1063-71. [Crossref] [PubMed]
- Fischlein T, Caporali E, Folliguet T, et al. Randomized controlled trial between conventional versus sutureless bioprostheses for aortic valve replacement: Impact of mini and full sternotomy access at 1-year follow-up. Int J Cardiol 2022;368:56-61. [Crossref] [PubMed]
- Miceli A, Gilmanov D, Murzi M, et al. Minimally invasive aortic valve replacement with a sutureless valve through a right anterior mini-thoracotomy versus transcatheter aortic valve implantation in high-risk patients. Eur J Cardiothorac Surg 2016;49:960-5. [Crossref] [PubMed]
Cite this article as: Allen K, El-Sherbini AH, El-Diasty M. The future of sutureless valve technology. J Vis Surg 2023;9:51.