
Case series: surgery for complications following aortic coarctation repair
Highlight box
Key findings
• Late complications of a successfully repaired aortic coarctation are not rare and the implications can be devastating.
• Referral to a specialised aortic centre for surveillance and expedited treatment is paramount for success.
What is known and what is new?
• Incidence of late complications is well known and variable depending on the primary repair technique.
• We suggest a more frequent follow-up protocol with agreed imaging interval for these patients.
What is the implication and what should change now?
• A more frequent imaging interval than the currently suggested by the guidelines is advised for those repaired in the adult life to be able to identify these cases before devastating consequences.
Introduction
Background
Coarctation of the aorta (CoA) accounts for 5–7% of congenital heart defects with an incidence of 0.3/1,000 births. It can be limited to the aortic isthmus or also affect the aortic arch. Associated cardiac pathologies include bicuspid aortic valve (BAV), ventricular septal defect (VSD), patent ductus arteriosus, transposition of the great arteries, atrioventricular canal defects and hypoplastic left heart syndrome (1,2).
Therapeutic interventions include open surgery (via median sternotomy or left thoracotomy) and balloon angioplasty with stent placement. The former is preferred in newborn and infants, whereas endovascular treatments are reserved for adolescents and adults. Surgical interventions have evolved from end-to-end anastomosis, subclavian flap, and patch aortoplasty to extended end-to-end anastomosis and interposition graft (1-3).
Today, most CoA are diagnosed and treated in the neonatal period. A late diagnosis and treatment are risk factors for established hypertension that may affect and increase the risk of late complications. Long-term complications include re-stenosis and aneurysm formation, with a life-time risk between 1–38% depending on the primary type of repair (1-3).
Those complications can be life-threatening due to the risk of aortic rupture, especially when undiagnosed. Optimal management includes discussion on a specialised multidisciplinary meeting and management in a dedicated aortovascular unit to minimise morbidity and mortality.
Objective and methods
We aim to review retrospectively in a case series format all consecutive patients presenting with late complications of a previous CoA repair that required second open surgery in our institution from April 2015 to January 2022. Our institution has the largest cardiothoracic surgery unit in the country as well as a Grown-Up Congenital Heart (GUCH) disease unit accepting direct referrals and consults from remote areas within the country.
Demographics, time from prior surgery to complication, clinical presentation, surgical techniques, and outcomes are analysed. Anonymised data was collected from our institutional database (Patient Tracking System, Dendrite Clinical Systems) and diagnostic images were obtained from our computerized tomography (CT) scan local server (Sectra PACS). Continuous variables are expressed as mean (range) +/− standard deviation as or sample followed a normal distribution.
Follow-up with clinical visits and serial CT aortograms scheduled prior to discharge, at 3, 6, 12 months and then annually has been maintained in our institution for all the patients.
Surgical techniques
Patients undergoing re-do descending thoracic aorta (DTA) replacement were approached via re-do left posterolateral thoracotomy through the 6th intercostal space after positioning in right lateral decubitus with arm flexed at 90°and hips tilted at 30°. The following monitoring systems and adjuncts are inserted: double lumen endotracheal tube; right radial (RRA) and right femoral arterial (RFA) lines; central venous line; electrocardiogram (ECG) leads; nasopharyngeal temperature probe; transoesophageal probe; urinary catheter; Vascath via right femoral vein connected to a rapid infusion system; near infra-red spectrometry (NIRS) electrodes located in the forehead, lumbar paraspinal region and bilateral calves; when time permitted, a spinal drain and moto-evoked potential (MEP) pin electrodes in the skull and four limbs are inserted for spinal cord protection and monitoring.
After exposure of the aorta, a tape is placed around the aorta at the clamping sites. We tend to perform these cases with left heart bypass (LHB) and sequential clamping unless hypothermic circulatory arrest (CA) is mandated by a large distal arch/proximal DTA aneurysm that contraindicates clamping the aorta at that location. LHB circuit is used to control haemodynamic disturbances secondary to changes in cardiac afterload after proximal aortic cross-clamping, reducing proximal hypertension and cardiac afterload and providing distal perfusion. Our preferred setting includes cannulation of the left inferior pulmonary vein with a 28 Fr cannula to drain oxygenated blood that will be reinfused by the centrifugal pump into the distal aorta/left femoral artery (LFA) via a 20 Fr cannula. Moderate systemic heparinisation [activated clotting time (ACT) target 300–350 s] is used and temperature is allowed to drift to 34 ℃. LHB flows are maintained between 1.5–2.5 L/min to keep the distal mean arterial pressure (MAP) >60 mmHg. Unless there is a documented drop in MEP signals during the clamping, we do not advocate for reimplantation of intercostal arteries in this scenario as the segment replaced is short and the spine will be perfused by collaterals.
Arch/frozen elephant trunk (FET) cases were done via median sternotomy and the patient in decubitus supine. In preparation for surgery, the following monitoring systems and adjuncts are inserted: endotracheal tube; RRA and RFA catheters; central venous line; ECG leads; nasopharyngeal temperature probe; transoesophageal probe; urinary catheter with integrated temperature probe; NIRS electrodes in the forehead and bilateral calves; when time permitted, a spinal drain is inserted for spinal cord protection.
Cardiopulmonary bypass (CPB) is established by cannulation of the distal ascending aorta and the right atrium; drainage of the left ventricle is achieved by cannulation of the right inferior pulmonary vein. Temperature is cooled to 22 ℃, allowing the decompressed heart to fibrillate spontaneously during cooling. At the desired temperature and after administration of cardioplegia, the circulation is discontinued and the aorta transected, exposing longitudinally the arch. We advocate for constructing the distal anastomosis in Zone 2 of the arch. Cerebral protection is achieved by administration continuous antegrade cerebral perfusion with cold blood at rates 1ml/kg/min of and targets of 55–60 mmHg pressure via selective cannulation of the innominate, left common carotid and left subclavian artery (LSA). A previously sized hybrid Plexus Thoraflex prosthesis (Terumo Aortic, Glasgow, Scotland) is then deployed antegradely under direct vision in the DTA. The distal anastomosis is performed with two layered 3/0 polypropylene suture. A 20 Fr arterial cannula is then inserted via the perfusion side arm of the prosthesis and after de-airing the distal circulation is restarted at progressive flows. Proximal anastomosis to the ascending aorta is then constructed in a same manner and the cross clamp (XC) removed. Finally, the individual supra-aortic trunks are anastomosed to the corresponding branches of the dacron prosthesis with continuous 5/0 polypropylene suture.
Immediate postoperative care
Maintaining haemodynamic stability with a desired targeted MAP >85 mmHg is key for the visceral and spinal protection; hence permissive use of vasoconstrictors (noradrenaline and vasopressin). We target MAPs >85 mmHg for the first week, and >75 mmHg for at least another week, to minimize delayed-onset paraplegia.
The cerebrospinal fluid (CSF) drainage (pressure set at 10 mmHg and volume removal of 20 mL/h) is continued for at least 2–3 days to minimize delayed-onset paraplegia. Other adjuncts for spinal protection include maintaining haemoglobin levels >9 mg/dL and oxygen saturations >95% to maximise oxygen delivery. We present this article in accordance with the PROCESS reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-12/rc).
Case presentation
From April 2015 to January 2022, nine patients required repair of a late complication from prior CoA repaired at different ages.
During the same period, two native CoA were treated in our institution with an interposition graft via left thoracotomy and another two were treated with an extra-anatomical bypass from the ascending to the descending aorta via median sternotomy during the emergency repair of a type A aortic dissection.
Mean age at time of primary CoA repair was 15.1±11.4 (range, 0–30) years; 67% of patients were male. Type of repair varied from patch repair (n=3), end-to-end anastomosis (n=2), interposition graft (n=2), patch and LSA reimplantation (n=1) and/or unknown (n=1). One patient who had an end-to-end repair as newborn had a balloon angioplasty at the age of 1 year. Concomitant cardiac conditions included: BAV (n=2), VSD (n=1). Three patients had associated syndromes (Turner’s, Kartagener and Antiphospholipid). Four patients were treated for established hypertension in the adult age (Table 1).
Table 1
| Patient number | Age CoA repair (years) | Sex | Type of repair | Concomitant lesions | Hypertension | Age complication (years) | Presentation | Diagnosis | Site of lesion | Size aorta (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | Male | Patch | No | 39 | Asymptomatic | Aneurysm | Proximal DTA | 44 | |
| 2 | 7 | Male | Patch | No | 22 | Haemoptysis → haemothorax | Contained rupture | Patch dehiscence | 26 | |
| 3 | 28 | Female | Interposition graft | Antiphospholipid syndrome | No | 37 | Chest pain | Contained rupture | Dehiscence distal anastomosis | 67 |
| 4 | 28 | Female | Interposition graft | Bicuspid aortic valve | Yes | 55 | Chest pain | Pseudoaneurysm | Dehiscence proximal anastomosis | 42 |
| 5 | 30 | Male | Unknown | Atrial fibrillation; AVR + MVR | Yes | 48 | Chest pain | Aneurysm; residual CoA distal LSA | Whole aorta | 90 |
| 6 | 5 | Male | End to end | No | 43 | Asymptomatic | Aneurysm | Proximal DTA involving LSA | 36 | |
| 7 | Newborn and 1 | Male | End to end + balloon angioplasty | Kartagener Sd; VSD repair | No | 33 | Asymptomatic | Pseudoaneurysm | Proximal DTA | 40 |
| 8 | 5 | Female | Patch | Bicuspid aortic valve | Yes | 48 | Hoarse voice | Aneurysm; residual CoA mid arch | Proximal DTA | 55 |
| 9 | 25 | Male | Patch + LSA reimplantation | Yes | 40 | Haemoptysis | Contained rupture | Mid DTA | 30 |
CoA, coarctation of the aorta; DTA, descending thoracic aorta; AVR, aortic valve replacement; MVR, mitral valve replacement; LSA, left subclavian artery; Sd, syndrome; VSD, ventricular septal defect.
Mean time to presentation with a late complication mandating re-do surgery was 28.7±11.3 (range, 9–48) years, with mean age at the second surgery being 43.8±10.8 (range, 22–65) years.
Clinical presentation included acute chest or back pain (3, 34%), haemoptysis (2, 22%), hoarse voice (1, 11%) or asymptomatic (3, 34%). Those who were asymptomatic, had been under regular surveillance in the GUCH service and were referred for consideration of surgery based on size criteria (distal arch >55 mm, DTA >60 mm, LSA >30 mm and/or documented expansion >3 mm/year). Diagnosis was made by contrast enhanced CT aortogram and varied from aneurysm of a distant location (Figures 1,2) to pseudoaneurysm in the previous CoA repair site with or without contained rupture (Figures 3-6). Mean maximum DTA size was 47.8 (range, 26–90) mm (Table 1).
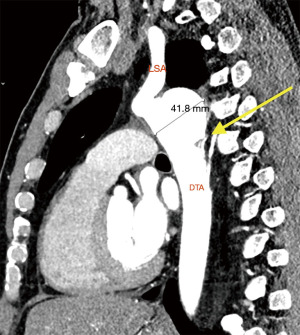
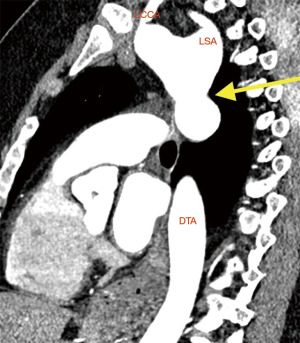
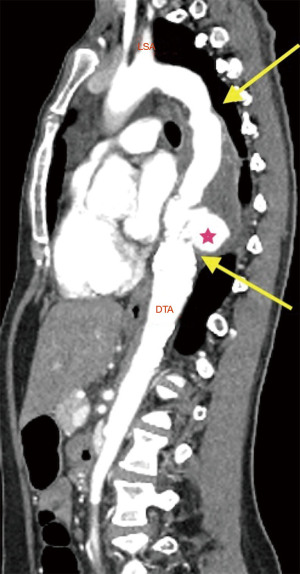
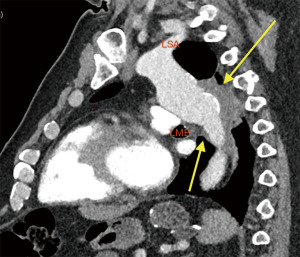
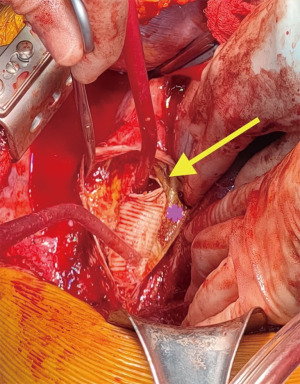
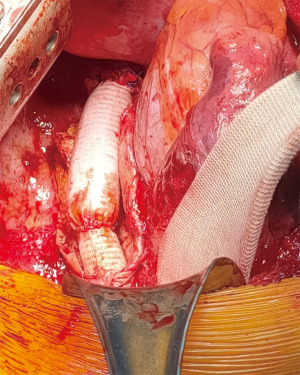
Note that the patients who presented with elective aneurysms had been under annual surveillance in our institution (n=4). Another patient, who presented with a pseudoaneurysm and impeding rupture was under 3-year imaging surveillance (Figure 5) and the remaining 4 patients were not under any surveillance program.
Timing for surgical repair was elective (4, 44%), urgent (2, 22%) and emergency (3, 34%). Most of the cases (7, 78%) had re-do DTA replacement via left thoracotomy, 2 cases (22%) had arch replacement with FET coverage of the CoA site. Rationale for arch and FET repair instead of re-do DTA was concomitant distal arch and LSA aneurysm and/or contained rupture of DTA with challenging patient anatomy for re-do left thoracotomy (morbid obesity >130 kg) (Table 2).
Table 2
| Patient number | Operation performed | Timing of surgery | Spinal protection | Temperature (℃) | Circulatory support/cannulation | Proximal clamp area | Ischemic times (min) | Complications |
|---|---|---|---|---|---|---|---|---|
| 1 | Re-do DTA | Elective | Spinal drain | 34 | LHB (LIPV/DTA) | Proximal to LSA | 77 | None |
| 2 | Re-do DTA | Emergency | None | 20 | CPB (LFA/LFV) | No clamp | CPB, 287; CA, 35 | Stroke |
| 3 | Re-do DTA | Emergency | Spinal drain | 34 | LHB (LIPV/LFA) | Distal to LSA | 69 | None |
| 4 | Re-do DTA | Urgent | Spinal drain + MEP | 34 | LHB (LIPV/DTA) | Proximal to LSA | 96 | Haemofiltration |
| 5 | Re-do DTA | Urgent | Spinal drain | 34 | LHB (LIPV/LFA) | Distal to LSA | 106 | Tracheostomy |
| 6 | Re-do DTA + replacement LSA | Elective | Spinal drain | 34 | LHB (LIPV/DTA) | Proximal to LSA | 127 | None |
| 7 | Re-do DTA | Elective | Spinal drain + MEP | 34 | LHB (LIPV/DTA) | Proximal to LSA | 135 | Reoperation for bleeding |
| 8 | Arch/FET | Elective | None | 22 | CPB (AA/RA) | N/A | CPB, 382; AXC, 230; CA, 230 | Reoperation for bleeding |
| 9 | Arch/FET | Emergency | Spinal drain | 22 | CPB (AA/RA) | N/A | CPB, 342; AXC, 118; CA, 100 | Endovascular closure of LSA |
CoA, coarctation of the aorta; DTA, descending thoracic aorta; LHB, left heart bypass; LIPV, left inferior pulmonary vein; LSA, left subclavian artery; CPB, cardiopulmonary bypass; LFA, left femoral artery; LFV, left femoral vein; CA, circulatory arrest; MEP, motor evoked potentials; FET, frozen elephant trunk; AA, ascending aorta; RA, right atrium; N/A, not applicable; AXC, aortic cross clamp.
Patients undergoing re-do DTA replacement were approached as described in the methods (Figures 5,6). Seven patients had spinal drain insertion for spinal cord protection and 2 patients had intraoperative MEP monitoring. The proximal clamp at the aorta was applied distal to the LSA in 4 cases and between the carotid and LSA in 2 cases. Six cases were done with LHB and mean intercostal ischemic times were 101.7 (range, 69–135) min and in the 2 cases that had LSA ischemia mean times were 21.5 (range, 17–26) min. The remaining re-do DTA case was performed under CPB via cannulation of both left femoral vessels prior opening of the chest as the patient presented with aortic rupture and haemothorax. Circulation was cooled down to 20° and the proximal anastomosis was done under 35 min of CA. Total CPB time was 287 min (Table 2).
Arch/FET cases were done as described in methods. One patient had spinal drain prior to surgery. Mean times were as follows: CPB 362 min, XC 124 min and CA 98 min.
Postoperative complications included: stroke (1, 11%), haemofiltration (1, 11%), tracheostomy (1, 11%), reintervention for bleeding (2, 22%) and temporary recurrent laryngeal nerve palsy (1, 11%). No paraplegia or mortality occurred in our series (Table 2).
All symptoms disappeared immediately after surgery and all patients remain alive and under regular follow-up with clinical visits and radiological surveillance at 3, 6, 12 months and then annually. Median follow-up is 3.1 (range, 0.8–5.6) years.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written consent was obtained from all the participants prior to surgery, authorising the use of their anonymised data and images for academic and research purposes. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
CoA is considered as part of a generalized arteriopathy and not only as narrowing of the aorta. It occurs either as a short stenosis at the level of the ductus arteriosus insertion or as a long hypoplastic arch segment. It is associated with BAV (up to 85%), ascending aorta aneurysms, sub or supra-aortic stenosis, mitral valve stenosis and other complex congenital defects and syndromes such as Turner’s (1,2).
The CoA results in increased cardiac afterload and upper body hypertension with hypoperfusion to the lower body [diagnosed as blood pressure (BP) gradient >20 mmHg, weak distal pulses, radiofemoral pulse delay or palpable collaterals] (4). Clinical presentation is determined by the severity of the coarctation, ranging from a critically unwell child to an asymptomatic adult. Beyond adolescence, the long-term survival is up to 60 years, however long-term morbidity is common related to aortic complications and the effects associated with longstanding hypertension (1-6).
Indication for intervention in adults is systemic hypertension with an upper to lower body pressure difference of >20 mmHg (1,2). Stenting has become the preferred treatment, providing there is appropriate anatomy (7-9). In children, indication for intervention is met if there is >20 mmHg gradient even in the absence of hypertension and/or if the CoA is duct dependent. In the presence of hypertension, the pressure difference of >20 mmHg is not a requirement if there is at least 50% narrowing relative to the aortic diameter (1,2).
Treatment of coarctation usually involves surgical or endovascular remodelling of the stenosed aortic segment. Although balloon angioplasty is safe and has high success rates in infants between 1–6 months, stent placement is not recommended in patients less than 25 kg due to small aortic size and potential injury to femoral artery. In younger patients, surgery is the preferred treatment method which can be carried out with low mortality of 1% (10). In adults, the randomised stent trials have shown the safety and effectiveness of endovascular treatment of coarctation up to 5 years however longer follow up will be required to determine late complications (9). We did not include any patients who had undergone endovascular therapy as an adult, but this could represent a selection bias, as those cases would likely be treated by endovascular specialists rather than open aortic surgeons.
Surgical techniques available include resection and end-to end anastomosis, patch aortoplasty, subclavian flap aortoplasty, extended end-to-end anastomosis and interposition graft (3).
Described complications of CoA repair include re-coarctation (10–41%), aneurysm (5–51%) and false aneurysm formation, endoleaks from endovascular repair and persistent hypertension requiring re-intervention (5). Patch aortoplasty may lead to higher rate of aneurysm formation (11,12), while interposition grafts are more prone to false aneurysms (3).
Recoarctation is most commonly attributable to insufficient aortic wall growth at the site of repair before the aorta has reached adult size and is therefore most common in patients who are younger at the time of operation, this is usually treated by endovascular therapy, with success rate reported as 94–97%. Complications of endovascular treatment include access site bleeding (3%), aortic dissection/rupture (2–5%), stent migration (6%), embolism (3%) (3,8,9).
As we have seen in this series, the most common complication requiring surgical management is aneurysm/pseudoaneurysm formation. This often occurs around the coarctation site in the context of abnormal aortic tissue if it was not entirely resected during initial surgical repair however may occur at other sites of native aorta (13). We use current size criteria for native aortic aneurysm of 5.5 cm in the arch and 6 cm in the DTA or any expansion in diameter >3 mm/year for true aneurysms and any size for false aneurysms (14).
Endovascular treatment is also an option to treat aneurysm/pseudoaneurysms, but requires and appropriate landing zone, not always present due to the proximity of the aortic arch and left subclavian. In equipoise between the two techniques, we guide therapy by age and comorbidities of the patients, leaning towards open surgery for young patients as these series report.
Given the high late complications rates necessitating reintervention in 5–50% of the cases, re-do repair of CoA represents an important field in aortic surgery. Complications following repair of CoA can occur late and patients may remain asymptomatic, therefore careful life-long congenital cardiology monitoring and surveillance is essential for these patients. The Mayo Clinic describe reintervention rate of 25% at 30 years including both the aorta, aortic valve or other cardiac surgery (12).
All patients require regular follow-up at least every year to monitor symptoms and BP status. Imaging of the whole aorta, preferably with cardiovascular magnetic resonance (CMR) is required to document post repair anatomy and complications (restenosis, aneurysm); current recommended imaging intervals are every 3–5 years (15), although as demonstrated in our cohort, this might not suffice to detect the false aneurysms in time before they become complicated with impeding and/or contained ruptures (16). It is important that all CoA treated patients are followed up and imaging is performed regularly, and in certain risk groups even yearly imaging in adulthood may be necessary.
We describe two different surgical approaches to address re-do CoA complications. The choice depends on the main concerning area (DTA vs. distal arch) and the feasibility to re-entry the left chest safely. Re-do DTA can be done with CPB but we preferred LHB as associates lower coagulopathy rates due to the lesser heparinisation dose but requires experienced aortic teams. Arch/FET with complete coverage of the abnormal area in the DTA, requires circulatory arrest with increased risk of stroke. Both re-do DTA and FET procedures carry risk of spinal cord ischemia and paraplegia (5–10%), hence why intraoperative monitoring and protection as well as robust postoperative management are paramount for success of these cases.
Our paper has the limitations of being a case-series with small number of patients. There is also a selection bias as we are unable to report the number of patients who are successfully repaired without complications or those repaired by endovascular methods.
Conclusions
Surgery for late complications of CoA repair can be successfully carried out with low morbidity by experienced aortic teams with standardised perioperative management. Standardised lifelong surveillance of CoA may allow early detection of complications and planning for elective surgery. Given the high rate and life-threatening nature of late complications, the need for life-long follow-up cannot be over-emphasized; however, this is not always available or adequate.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PROCESS reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-12/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-12/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-12/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written consent was obtained from all the participants prior to surgery, authorising the use of their anonymised data and images for academic and research purposes. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 2021;42:563-645. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008;118:e714-833. [PubMed]
- Beckmann E, Jassar AS. Coarctation repair-redo challenges in the adults: what to do? J Vis Surg 2018;4:76. [Crossref] [PubMed]
- Hijazi ZM. Catheter intervention for adult aortic coarctation: be very careful! Catheter Cardiovasc Interv 2003;59:536-7. [Crossref] [PubMed]
- Lee MGY, Babu-Narayan SV, Kempny A, et al. Long-term mortality and cardiovascular burden for adult survivors of coarctation of the aorta. Heart 2019;105:1190-6. [Crossref] [PubMed]
- Meijs TA, Minderhoud SCS, Muller SA, et al. Cardiovascular Morbidity and Mortality in Adult Patients With Repaired Aortic Coarctation. J Am Heart Assoc 2021;10:e023199. [Crossref] [PubMed]
- Holzer RJ, Gauvreau K, McEnaney K, et al. Long-Term Outcomes of the Coarctation of the Aorta Stent Trials. Circ Cardiovasc Interv 2021;14:e010308. [Crossref] [PubMed]
- Galiñanes EL, Krajcer Z. Most Coarctations, Recoarctations, and Coarctation-Related Aneurysms Should Be Treated Endovascularly. Aorta (Stamford) 2015;3:136-9. [Crossref] [PubMed]
- García-Pavía P, Goicolea Ruigómez J, López-Mínguez JR, et al. Endovascular treatment of long-term complications following surgical repair of aortic coarctation. Rev Esp Cardiol 2010;63:473-7. [PubMed]
- Sakurai T, Stickley J, Stümper O, et al. Repair of isolated aortic coarctation over two decades: impact of surgical approach and associated arch hypoplasia. Interact Cardiovasc Thorac Surg 2012;15:865-70. [Crossref] [PubMed]
- Walhout RJ, Lekkerkerker JC, Oron GH, et al. Comparison of polytetrafluoroethylene patch aortoplasty and end-to-end anastomosis for coarctation of the aorta. J Thorac Cardiovasc Surg 2003;126:521-8. [Crossref] [PubMed]
- Brown ML, Burkhart HM, Connolly HM, et al. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol 2013;62:1020-5. [Crossref] [PubMed]
- Roselli EE, Qureshi A, Idrees J, et al. Open, hybrid, and endovascular treatment for aortic coarctation and postrepair aneurysm in adolescents and adults. Ann Thorac Surg 2012;94:751-6; discussion 757-8. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 2021;42:563-645. [Crossref] [PubMed]
- Somers T, Nies HMJM, van Kimmenade RRJ, et al. Necessity of life-long follow-up after surgery for coarctation of the aorta: a case series of very late false aneurysm formation. Eur Heart J Case Rep 2022;6:ytac073. [Crossref] [PubMed]
Cite this article as: Olayiwola A, Hara N, Yates MT, Lopez-Marco A. Case series: surgery for complications following aortic coarctation repair. J Vis Surg 2023;9:50.


