
Robotic uncinate enucleation with transmesenteric sleeve duodenectomy: a novel approach
Highlight box
Surgical highlights
• Transmesenteric approach to enucleation of tumors of the uncinate process.
What is conventional and what is novel/modified?
• Enucleation of benign pancreatic tumors via an open approach is conventional.
• Robotic pancreatic enucleation of the pancreatic uncinate process utilizing a transmesenteric approach is novel.
What is the implication, and what should change now?
• Benign tumors of the pancreas can be resected in a minimally invasive fashion even when in a difficult location such as the uncinate process.
Introduction
Background
Enucleation of pancreatic tumors is an organ preservation technique in that it can preserve normal pancreatic parenchyma, reduce the risk of endocrine and exocrine insufficiency and decrease morbidity from a formal pancreatic resection (1,2). Indications include benign and low-grade neuroendocrine tumors of the pancreas (3). For lesions located anteriorly on the pancreas, enucleation can be performed easily using either open or minimally invasive techniques (4). Notably, patients who undergo these open approaches have increased postoperative pain and longer hospitalization times. These lesions on the anterior pancreas are conventionally accessed through a supramesocolic approach after opening the gastrocolic ligament and gaining access to the lesser sac where the pancreas is readily visible (5).
Rationale
This technique, however, becomes much more difficult when the lesion is located in the uncinate process where the duodenum and mesenteric vasculature restrict access to this portion of the pancreas (6). The uncinate process is a part of the pancreas that bends backwards and beneath the body of the pancreas in which the superior mesenteric artery and the superior mesenteric vein (SMV) intersect.
Objective
Herein, we describe a novel approach to robotic pancreatic enucleation of the uncinate process using a transmesenteric technique (Video 1). An associated sleeve duodenectomy, which is resection of the duodenum in a sleeve fashion to allow for jejunal anastomosis, is also for reconstruction purposes. We present this article in accordance with the SUPER reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-22/rc).
Preoperative preparations and requirements
Patient selection and workup
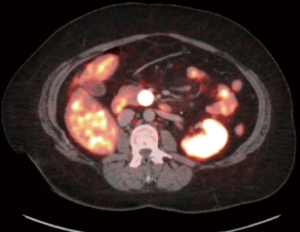
A 44-year-old female presented with diarrhea. A 2 cm lesion of the uncinate process of the pancreas was identified on computerized tomography (CT) imaging. The lesion was positive on 8Ga-DOTATATE positron emission tomography (PET)/CT scan as seen in Figure 1. Biopsy showed a low grade pancreatic neuroendocrine tumor (PNET), Ki-67 <3%, and normal chromogranin A level.
Equipment preference card
All robotic cases are performed using the da Vinci Xi® robotic platform (Intuitive Surgical, Sunnyvale, CA, USA). Robotic instruments include monopolar curved scissors, fenestrated bipolar forceps, a vessel sealer device, and PrograspTM forceps (Intuitive Surgical, Sunnyvale, CA, USA). The large needle driver is used for suturing. Monocryl® and V-LocTM sutures (Medtronic, Minneapolis, MN, USA) are used for anastomoses and closure of the intestines, respectively.
Ethical standard
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript, the accompanying image, and the video. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step description
Preparation
This operation must be performed in a standard operating room with robotic equipment. A surgeon, an anesthesiologist, a surgical technician, and an operative nurse must be present for the procedure to take place. The patient is taken to the operating room and placed in the supine position. General anesthesia is induced, and an arterial line and two peripheral intravenous lines are placed. A nasogastric tube and urinary catheter are placed along with sequential compression devices on the patient’s lower extremities. Both arms are extended out on arm boards for anesthesia access.
Access & visualization
Pneumoperitoneum is obtained with a Veress needle in an infraumbilical incision. An initial 8 mm robotic trocar is placed and laparoscopy is performed to confirm no peritoneal spread of disease. The patient is then placed into reverse Trendelenburg position for approximately 8–10 degrees. Four 8 mm robotic trocars are placed across the upper abdomen in a straight line, and the infra-umbilical port is changed to a 12 mm laparoscopic port for assistance. The robot is then docked, and the robotic arms and instruments are connected. Arm 1 carries the fenestrated bipolar and arm 2 is reserved for the robotic camera. Arm 4 carries the PrograspTM (Intuitive Surgical) for retraction and arm 3 is used as the “working port” where all instrument exchanges occur.
Dissection & enucleation
First to access the duodenum from a transmesenteric approach, the transverse colon is lifted cephalad. The bare area within the mesentery to the right of the middle colic vessels is identified and opened. The duodenum is then seen through this window. The duodenum can then be dissected from its retroperitoneal attachments and mobilized moving from lateral to medial exposing as much of the duodenum as possible until we begin to see the uncinate process towards the mesenteric vessels. To fully access the uncinate process in which the lesion is situated, the third and fourth portions of the duodenum and proximal jejunum require further mobilization, which ultimately leaves these portions of the duodenum devascularized. We move to the left of the middle colic vessels and identify the ligament of Treitz. The proximal jejunum is transected with a stapler and a vessel sealer dissects the mesentery and attachments to the ligament of Treitz. Once completely freed the duodenum and proximal jejunum are pulled behind the mesenteric vessels to the patient’s right side. This maneuver then allows for dissection of the uncinate process away from the duodenum as well as the SMV. Intraoperative ultrasound is then used to identify the tumor in the uncinate process. The enucleation is performed with monopolar scissors. This area tends to be quite vascular and may require suture ligation of bleeding vessels for hemostasis.
Reconstruction
The devascularized duodenum is then stapled at its proximal extent and prepared for reconstruction. The stapled end of jejunum is brought through the mesenteric window where the duodenal dissection was performed and positioned in an isoperistaltic fashion to create a side-to-side duodenojejunostomy. Prior to creating an anastomosis, the ampulla of Vater should be identified with either intraoperative ultrasound, direct visualization, or cannulation of the common bile duct with a catheter. The anastomosis is created with a robotic stapler in a side-to-side fashion and the common channel is closed using a running 4-0 V-LocTM suture. If the cystic duct is used to cannulate the bile duct for ampullary detection, the gallbladder should be removed. The specimen is placed in a retrieval sac and removed via the 15 mm port site.
Closure
A drain is placed near the anastomosis. Flowable hemostatic agents are used for hemostasis. The 15 mm incision is closed with an interrupted suture. The fascia of the 8 mm trocar sites is generally not closed. Operative time averages at 120 minutes.
Postoperative considerations and tasks
An upper gastrointestinal series (UGIs) was obtained on postoperative day one. In this patient, the UGI showed a patent anastomosis without leak or obstruction. The patient slowly advanced to a pureed diet which she continued for 2 weeks. The drain is checked for amylase levels on postoperative day 3 and removed if output is not amylase rich with no evidence of bile or chylous leak. Final pathology revealed a 2 cm, low grade neuroendocrine tumor with negative margins. Patients are typically discharged on postoperative day 3 with or without their surgical drain depending on amylase level to indicate leak or not. They follow up in 1 week for surgical site check and drain removal and in 1 month for another standard postoperative check. They heal quickly from their minimally invasive surgery and given the low-grade nature of their neuroendocrine tumor, no long-term follow-up is necessary.
Tips and pearls
- An assistant port is essential for passing suture and sponges as well as suctioning. We use a 15 mm trocar in this case, to also accommodate the robotic stapler cannula and robotic stapler which eliminates the need for a port larger than an 8 mm off midline requiring fascial closure at the end of the case.
- Place sponges under the liver to aid in retraction of the left lobe and exposure. This will obviate the need for additional liver retractors.
- The round ligament can be harvested as a well vascularized flap to cover the duodenotomy repair. This reinforces the repair and adds an extra layer of protection.
- Always use intraoperative ultrasound to identify vascular and ductal anatomy in comparison to the ampullary mass. This helps identify important anatomic landmarks for the resection and reconstruction along with avoiding inadvertent injuries.
- The ampulla of Vater must be identified prior to creation of an anastomosis. This can be done with either ultrasound, direct visualization through the duodenum or by cannulating the common bile duct with a catheter and feeding it through the ampulla. This helps avoid inadvertent injuries.
Discussion
Surgical highlights
The surgical highlight from this paper is performing a transmesenteric sleeve duodenectomy for robotic pancreatic enucleation of a benign tumor in pancreatic uncinate process.
Strengths and limitations
This paper provides an alternative approach to difficult to access benign tumors of the pancreas without needing to convert to an open procedure or perform a more invasive surgery such as a pancreaticoduodenectomy. Furthermore, the visualization of the uncinate via this technique is superior to other techniques. Limitations of this paper are that a surgeon needs to have experience with pancreas resections from an open and robotic perspective prior to attempting this technique. Pancreatic surgeons must be experts at this operation in the open approach before advancing to the robotic approach. Furthermore, some institutions may not have robotic access available to them, limiting their ability to exercise this technique.
Comparisons with other surgical techniques and research
Compared to open approaches, this robotic operation results in lower morbidity along with decreased length of stay as previously shown as an advantage of minimally invasive surgery. Furthermore, compared to laparoscopy, the wristed instruments of robotic surgery allow for easier dissection and resection.
Implications and actions recommended
Future studies are necessary to compare the outcomes and benefits to this robotic approach compared to laparoscopic and open approaches.
Conclusions
We report a case of a neuroendocrine tumor of the uncinate process of the pancreas which was able to be completely resected using a novel robotic approach to pancreatic enucleation. This allowed for a minimally invasive resection that otherwise may have required an open operation or pancreaticoduodenectomy.
Acknowledgments
The manuscript has been presented at Podium presentation at the Clinical Robotic Surgery Association (CRSA) World Congress in Rome, Italy, December 2022.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-22/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-22/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-22/coif). J.B.M. and D.V. are consultants for Intuitive Surgical. F.N.M.’s fellowship stipend is sponsored by Intuitive Surgical. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript, the accompanying image and the video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xu J, Li F, Zhan H, et al. Laparoscopic enucleation of pancreatic tumours: a single-institution experience of 66 cases. ANZ J Surg 2021;91:106-10. [Crossref] [PubMed]
- Zhou Y, Zhao M, Wu L, et al. Short- and long-term outcomes after enucleation of pancreatic tumors: An evidence-based assessment. Pancreatology 2016;16:1092-8. [Crossref] [PubMed]
- Beger HG, Poch B, Vasilescu C. Benign cystic neoplasm and endocrine tumours of the pancreas—when and how to operate—an overview. Int J Surg 2014;12:606-14. [Crossref] [PubMed]
- Lu WJ, Xu B, Gao SL, et al. Enucleation of benign or borderline pancreatic head tumors adjacent to the common pancreatic duct. Pancreas 2012;41:336-7. [Crossref] [PubMed]
- Nakao A. Isolated pancreatectomy using mesenteric approach. J Hepatobiliary Pancreat Sci 2022;29:293-300. [Crossref] [PubMed]
- Soejima Y, Toshima T, Motomura T, et al. Technical Feasibility and Oncological Legitimacy of Enucleation of Intraductal Papillary Mucinous Neoplasm Located at the Pancreatic Head or Uncinate Process. Anticancer Res 2017;37:321-6. [Crossref] [PubMed]
Cite this article as: Ricker AB, McCarron FN, Vrochides D, Martinie JB. Robotic uncinate enucleation with transmesenteric sleeve duodenectomy: a novel approach. J Vis Surg 2024;10:2.


