
Surgical coronary angioplasty with common femoral artery and coronary artery bypass grafting for the treatment of left main coronary artery aneurysm outside the stent: a case report
Highlight box
Key findings
• This case report describes the successful surgical management of a left main coronary artery (LMCA) aneurysm located outside the stent using coronary artery patch plasty with the common femoral artery (CFA) and coronary artery bypass grafting (CABG).
What is known and what is new?
• Surgical coronary artery plasty has been established as a valid treatment option for patients with symptomatic coronary artery aneurysms (CAAs). Various materials are utilized for patch plasty, yet the optimal choice remains under study.
• We report the innovative use of a CFA patch in coronary angioplasty for managing a CAA outside the stent in the LMCA. This expands the therapeutic options, although further research is required to validate its safety and efficacy on a larger scale. Implication: The application of CFA in surgical coronary angioplasty, coupled with CABG, could represent a valuable strategy for managing LMCA aneurysms outside stents.
What is the implication, and what should change now?
• The application of CFA in surgical coronary angioplasty, coupled with CABG, could represent a valuable strategy for managing LMCA aneurysms outside stents.
Introduction
Coronary artery aneurysms (CAAs), particularly those in the left main coronary artery (LMCA), are uncommon clinical entities (1,2) that can pose significant diagnostic and management challenges. Treatment options are not well-defined due to the lack of extensive research data and the anatomical complexities of these aneurysms (1,3,4). In this report, we present an unusual case of an expanding symptomatic CAA in the LMCA outside the stent. The successful management was achieved using coronary artery patch plasty with a patch from the common femoral artery (CFA), a novel approach, in conjunction with coronary artery bypass grafting (CABG). This case introduces the potential of the CFA patch in coronary angioplasty, especially in complex cases involving CAAs outside stents, adding valuable insight to this limited area of research. We present this case in accordance with the CARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-10/rc).
Case presentation
Case (Figures 1,2, Video 1)
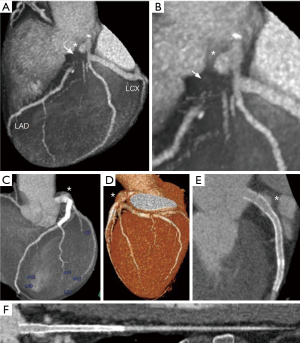
A 51-year-old Japanese male presented with exertional chest pain during flat-ground walking for the past three months. He had visited a hospital earlier, where electrocardiographic changes were noted, leading to a referral to our hospital for further evaluation. T-wave flattening was observed in leads I, aVL, and V5–6. The patient had a family history of angina in his brother. Cardiac computed tomography angiography (CTA) revealed a large aneurysm in the LMCA and suspected occlusion in the proximal left anterior descending artery (LAD), along with possible stenosis in the left circumflex artery (LCX) (Figure 1A,1B).
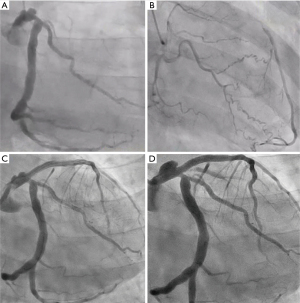
Consequently, a diagnosis of effort angina was made, and the patient was admitted for investigation. Cardiac catheterization revealed a giant ulcerated CAA in LMCA, total occlusion of the proximal LAD, and 75% stenosis in the LCX identified (Figure 2A). Collateral circulation to the distal LAD was noted from the right coronary artery and the distal branches of the circumflex artery (Figure 2B). The patient underwent percutaneous coronary intervention (PCI) for the chronic occlusive lesion in the LAD artery, including directional coronary atherectomy (DCA) performed for the proximal LAD occlusion, followed by stent placement from LMCA entrance to the mid-LAD, using an everolimus-eluting stent (Figure 2C). Dual antiplatelet therapy (DAPT) with aspirin and clopidogrel, as well as statin therapy, were initiated. Post-PCI, the patient experienced relief from chest symptoms. However, after one year, the patient discontinued outpatient visits and medication. Three years after the PCI, the patient presented to the hospital with chest discomfort, dyspnea, and nausea during walking for his daily commute. Cardiac CTA showed enlargement of the CAA of the LMCA outside the stent but no stenosis within the stent, but LCX appeared jailed by the stent, suggesting possible stenosis (Figure 1C-1F).
Consequently, the patient was admitted for further evaluation. On the same day, catheterization revealed no new stenotic lesions in the stent or native coronary artery. However, an enlargement of the pre-existing massive ulcer/aneurysmal lesion outside the stent in LMCA was noted (Figure 2D). Plaque rupture at the site of the CAA in LMCA was suspected of causing the current episode of unstable angina. The case was discussed with the heart team. Considering the patient’s young age and the need to prevent future cardiovascular events, the intervention was deemed necessary. Since a covered stent that could adequately fit the large lesion was unavailable, surgical treatment was deemed appropriate.
Surgery (Figure 3, Video 2)
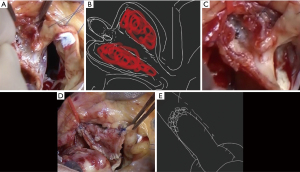
Under general anesthesia, a median sternotomy was performed, and the CFA and great saphenous vein were obtained from the right groin and thigh, respectively. The CFA, which had a diameter of 1.2 cm, was harvested at a length of 4.5 cm and substituted with an 8 mm expanded polytetrafluoroetylene/polyethylene terephthalate (PTFE/PET) prosthetic graft Fusion (Getinge, Gothenburg, Sweden). The excised CFA was opened longitudinally to create a 4.5 cm × 4 cm plate used as a patch. Left internal thoracic artery (LITA) was harvested. After systemic heparinization, cardiopulmonary bypass was initiated with aorto-bicaval cannulation. Retrograde cardioplegia induced cardiac arrest and the ascending aorta was cross-clamped. Distal anastomosis of saphenous vein graft (SVG) was made with the obtuse marginal (OM) branch of the LCX. The left coronary artery (LCA) ostium was exposed by transecting the ascending aorta at the level of the sinotubular junction (STJ). Examination of the LCA ostium from within the aortic lumen revealed a stent protruding through the ostium. After dissecting the left coronary sinus, an aneurysm of LMCA was detected, and the left coronary sinus was opened towards the LCA ostium. The LCA was then opened, beginning over the stents from the LMCA to the LAD. A pale red thrombus was observed in the aneurysm of the LMCA (Figure 3A,3B). The stents in the LAD were longitudinally opened, and all but the section of the stent covering the ostium of LCX was extracted along with a thin membrane (Figure 3C).
The CFA patch, measuring 4.5 cm × 4 cm, was sutured from the distal side of the coronary opening of the LAD towards the LMCA with a running suture utilizing 6-0 monofilament. The patch with the aortic wall was then continued with a 5-0 monofilament running suture (Figure 3D). The most distal part of the CFA patch with the LAD opening was intentionally constricted using five buttressed U-stay sutures with autologous pericardium on the patch side and felt pledget on the LAD side (Figure 3E). Subsequently, CABG was performed, including the anastomosis of LITA to the mid-LAD. After the reconstruction of the ascending aorta, SVG was anastomosed in the ascending aorta. After administering a hot shot through the root cannula, the cross-clamp was released. The graft flow was measured at LITA-LAD flow 5.1/pulsatility index (PI) 10 and SVG-OM flow 4.4/PI 25.
Postoperative study (Video 3)
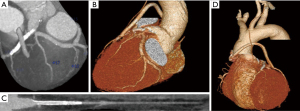
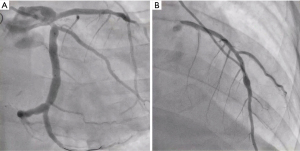
Postoperative cardiac CTA (Figure 4) and coronary angiography (CAG) (Figure 5) confirmed the success of coronary patch angioplasty and CAG. 3D reconstruction (Figure 4A,4B) and curved planner reconstruction (CPR) (Figure 4C) of CTA (Figure 4) revealed a 10 mm dilation at the angioplasty site of LMCA with CFA. All bypass grafts were patent; however, there was poor development of LITA to LAD bypass (Figure 4D). CAG (Figure 5A,5B) showed smooth vessel walls and patent grafts. The LITA-LAD bypass appeared to exhibit a to-and-fro flow pattern, suggesting it was competitive with the native flow of the LAD, which was found to be well-maintained.
Postoperative course
The patient was discharged in good postoperative condition on the eleventh day after the operation.
The patient remained symptom-free for 1 year and 9 months postoperatively.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report, accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This case report highlights the successful management of a symptomatic CAA located outside the stent in the LMCA using coronary patch plasty with CFA and CABG.
This case report presents a complex case for several reasons. Firstly, the aneurysm was located outside the stent, which posed a challenge for treatment. Additionally, the patient had an LMCA aneurysm with total occlusion of the LAD, which was treated with PCI using DCA and stenting that jailed the ostium of the LCX. Finally, there was the absence of in-stent restenosis.
Given these factors, the authors conducted coronary angioplasty of the LMCA using a CFA patch after removing the stents, except those overlaying the LCX ostium. Additionally, CABG was performed using LITA-LAD and an aorta-SVG (Ao-SVG)-LCX. The authors also employed additional sutures to induce stenosis in the LAD ostium to avoid flow competition.
Various materials can be used for coronary angioplasty patches, such as the saphenous vein, pericardium, internal thoracic artery, pulmonary artery, and superficial femoral artery (5-7). In this case report, the CFA was utilized as a patch for the first time. This decision was based on several factors, including the material’s availability, handling characteristics, and antithrombogenicity. Additionally, it was found to be easy to apply to the aortic wall and capable of withstanding arterial pressure, ensuring graft function with minimal degenerative changes compared to other options such as autologous pericardial patches or SVGs, which have a potential to fail at a later stage (5).
One of the most critical issues in coronary angioplasty involving the coronary ostium is patch kinking, which can disrupt coronary artery flow and lead to severe myocardial ischemia. Fortunately, the current technique allowed for satisfactory coronary artery enlargement without patch kinking and ensured adequate coronary artery flow. The autologous superficial femoral artery (6,7) and femoral artery homograft (8,9) have been reported to be successfully used for coronary ostial plasty in patients with Takayasu’s arteritis and in young adults with congenital heart disease, respectively. Nonetheless, it is essential to evaluate the aortic root and coronary arteries by monitoring the clinical course of patients treated with autologous CFA patch grafts in the coronary ostium and the sinus of Valsalva.
Because in-stent restenosis has not been observed, performing CABG on the distal segment of the coronary arteries is challenging due to flow competition between the native coronary arteries and the grafts (10,11). To mitigate this, we introduced sutures to induce stenosis in the LAD, which could aid in sustaining graft flow, especially in the early postoperative period. However, complete excision or ligation of the aneurysm with bypass may serve as an alternative option, although the failure of the LITA-LAD can be rare but hazardous in case the LMCA were to become occluded.
Given the increase in thrombogenicity in the early postoperative period and the exposed state of the CFA patch, we had concerns about the risk of thrombus formation and occlusion at the coronary aneurysm repair site. To mitigate this risk, we implemented intensified antithrombotic therapy postoperatively. Moreover, to prevent cardiovascular events during the early postoperative period when the risk of thrombotic occlusion is high, we performed CABG on the distal LAD, the repair site.
Although the patency of the LITA-LAD is good, it could be potentially fatal if an occlusion occurred, leading to a widespread myocardial infarction due to acute LAD occlusion. To avoid such a scenario, we performed CABG. Three months postoperatively, we expect the coronary artery repair site to be covered with neointima, reducing thrombogenicity. To ensure the patency of the bypass during this period, we intentionally created a stenosis on the central side of the LAD anastomosis site, which had no stenosis.
Postoperative imaging revealed competitive flow, which is a favorable result, but long-term follow-up is essential. Currently, one year and nine months have passed postoperatively, and the patient is symptom-free. If symptoms recur, we will consider evaluating with imaging. As for antithrombotic therapy, based on past experiences with coronary patch angioplasty (Taksnashi S. Personal communication. February 19, 2022), we plan to administer DAPT plus warfarin for three months postoperatively, DAPT for up to one year, and single antiplatelet therapy (SAPT) thereafter.
This case report’s findings should be interpreted in light of several limitations. Primarily, the report represents a single patient’s experience, which may not generalize to all patients with similar conditions. The longer-term outcomes and durability of the applied procedures, including using the femoral artery as a patch and CABG, are unknown and require further investigation. Deliberately constricting the LAD ostium to avoid flow competition also needs additional evaluation for its general applicability and potential complications. Hence, while the case offers valuable insights, larger studies and longer follow-up periods are essential for a comprehensive understanding and validation of these treatment strategies.
Conclusions
In conclusion, surgical coronary angioplasty with the CFA and coronary bypass grafting for an LMCA aneurysm outside the stent was successful. The CFA patch represents a promising option for coronary angioplasty patches, and sutures may be used to induce stenosis in the LAD ostium to mitigate flow competition. However, further research is necessary to confirm the safety and efficacy of this approach in larger patient populations.
Coronary patch angioplasty with CFA and coronary bypass grafting for an LMCA aneurysm outside the stent was successful.
Acknowledgments
This paper was presented at the 34th annual meeting of the Japanese Coronary Association, Tokyo, taking place between the 1st and 3rd of December, 2022.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-10/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-10/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-10/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report, accompanying images, and videos. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swaye PS, Fisher LD, Litwin P, et al. Aneurysmal coronary artery disease. Circulation 1983;67:134-8. [Crossref] [PubMed]
- Doustkami H, Maleki N, Tavosi Z. Left Main Coronary Artery Aneurysm. J Tehran Heart Cent 2016;11:41-5. [PubMed]
- Zhu X, Zhou Q, Tong S, et al. Challenges and strategies in the management of coronary artery aneurysms. Hellenic J Cardiol 2021;62:112-20. [Crossref] [PubMed]
- Alshehri AM. Large Aneurysm of the Left Main Coronary Artery: A Case Report. Saudi J Med Med Sci 2019;7:187-9. [Crossref] [PubMed]
- Harling L, Sepehripour AH, Ashrafian H, et al. Surgical patch angioplasty of the left main coronary artery. Eur J Cardiothorac Surg 2012;42:719-27. [Crossref] [PubMed]
- Oishi K, Arai H, Yoshida T, et al. Coronary Ostial Patch Angioplasty With Femoral Artery in Takayasu Arteritis. Ann Thorac Surg 2020;110:e427-30. [Crossref] [PubMed]
- Murashita T, Yoshimoto K, Sugiki H, et al. Bilateral coronary ostial patch angioplasty with autologous pericardium in Takayasu arteritis: a case requiring replacement of the aortic valve and ascending aorta. Eur J Cardiothorac Surg 2004;26:866-8. [Crossref] [PubMed]
- Mosca R, Chen D, Halpern D, et al. Femoral artery homograft for coronary artery plasty following arterial switch operation. JTCVS Tech 2020;4:232-4. [Crossref] [PubMed]
- Maeda K, Ryan KR. Commentary: A new option for patch material on coronary artery ostium plasty. JTCVS Tech 2020;4:235-6. [Crossref] [PubMed]
- Sabik JF 3rd, Lytle BW, Blackstone EH, et al. Does competitive flow reduce internal thoracic artery graft patency? Ann Thorac Surg 2003;76:1490-6; discussion 1497. [Crossref] [PubMed]
- Swillens A, De Witte M, Nordgaard H, et al. Effect of the degree of LAD stenosis on "competitive flow" and flow field characteristics in LIMA-to-LAD bypass surgery. Med Biol Eng Comput 2012;50:839-49. [Crossref] [PubMed]
Cite this article as: Nakao T, Tsuda S. Surgical coronary angioplasty with common femoral artery and coronary artery bypass grafting for the treatment of left main coronary artery aneurysm outside the stent: a case report. J Vis Surg 2024;10:5.


