The novel use of Perceval valve for pulmonary valve replacement in carcinoid syndrome: a case report
Highlight box
Key findings
• A Perceval bioprosthetic valve can be considered for the pulmonic valve position in patients where less dissection is desired.
• The use of a Perceval valve in such cases may be associated with shorter operative times, improved hemodynamics, and enhanced patient recovery.
What is known and what is new?
• Sutureless and rapid deployment valves have greatly influenced minimally invasive and complex valve surgery.
• This is the first case reporting the use of a Perceval valve in the pulmonic position for a patient with carcinoid syndrome.
What is the implication, and what should change now?
• Sutureless bioprosthetic valves should be considered for patients undergoing complex cardiac operations.
Introduction
Background
The advent of sutureless bioprosthetic valves has given surgeons additional options in managing patients with complex aortic valve disease. Potential benefits include facilitation of minimally invasive cardiac surgery, reduced operative times especially in complex cases, excellent hemodynamics, and potential benefits for catheter-based procedures in the future (1,2). These valves have also been shown to be effective in managing older patients and those with valvular insufficiency, small roots, and infective endocarditis (3,4).
Carcinoid syndrome refers to a constellation of symptoms caused by the release of humoral mediators from neuroendocrine tumors (NETs), such as polypeptides, prostaglandins, and biogenic amines (5). Common clinical presentations of carcinoid syndrome include flushing, diarrhea, fatigue, cognitive impairment, and cardiac symptoms consistent with right-sided heart failure (5). Cardiac involvement occurs in up to 60% to 70% of patients with NETs, and can cause plaque-like deposits of fibrous tissue on the endometrium, valves, chambers, pulmonary artery, and aorta (5).
Rationale and knowledge gap
Given the high surgical risk profile of this patient and the extent of the operation, a sutureless valve was chosen to reduce the amount of suturing required to mitigate the bleeding risk. To our knowledge, this is the only reported case of a Perceval sutureless valve ever being implanted in the pulmonary position in a patient with carcinoid syndrome.
Objective
Herein, we present the case of a patient with carcinoid syndrome who was found to have symptomatic tricuspid valve regurgitation (TR) and pulmonary valve stenosis and underwent tricuspid valve replacement (TVR) and pulmonary valve replacement (PVR) using a Perceval sutureless bioprosthetic valve (Corcym, London, UK). We present this article in accordance with the CARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-34/rc).
Case description
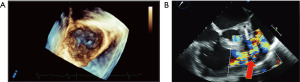
This is the case of a 64-year-old male of Southeast Asian descent who presented with anasarca and dyspnea (NYHA class IV) in the setting of unintentional weight loss over 3 months and worsening daily diarrhea and flushing over the past 18 months. Investigations included blood work, a computed tomography (CT) scan, flexible sigmoidoscopy, upper endoscopy, colonoscopy, and liver biopsy. His chromogranin A was 662 ng/mL. The liver biopsy confirmed carcinoid syndrome, while the CT scan showed a primary NET in the small bowel and metastases to the liver, pleura, and vertebral body. Metastases to the skin were also noted. Echocardiography also revealed a carcinoid tricuspid valve with severe TR, mild pulmonary valve stenosis secondary to all valve cusps being abnormally thickened (Figure 1A), severe pulmonary valve regurgitation (Figure 1B, red arrow), and moderate right ventricular (RV) dysfunction. Coronary angiography did not demonstrate flow-limiting coronary artery disease. His past medical history was otherwise remarkable for hypertension, gastroesophageal reflux disease, hearing loss, and gout. Given his class IV NHYA clinical status, the patient was started on the appropriate medical therapy for his carcinoid syndrome and was consented for high-risk double-valve surgery.
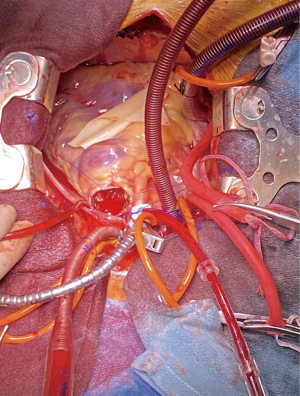
In January 2022, he underwent a full median sternotomy, followed by bicaval cannulation, and antegrade administration of del Nido cardioplegia. A 3-cm longitudinal incision was made in the right ventricular outflow tract (RVOT), and pulmonary valve inspection confirmed a stenotic carcinoid pulmonary valve. The native valve was excised and the RVOT, which was small, was irrigated with copious amounts of saline. Perceval valve sizers were used to measure the pulmonic valve annulus and a medium valve was chosen. Three guiding sutures were placed, allowing for the valve to be parachuted into the presumed annulus. The valve was ballooned at 4-atmosphere for 30 seconds. Direct visualization confirmed a well-seated Perceval valve in the pulmonic position (Figure 2). A small bovine patch was placed onto the RVOT, since it was small, and to avoid tension or any potential impingement on the valve cage. The tricuspid valve was then inspected, revealing a stenotic valve that was fibrosed. An attempt was made to repair the tricuspid valve, but it was deemed to be unrepairable due to extensive fibrosis. Therefore, a TVR with a 31-mm St. Jude Epic valve (Abbott, MN, USA) was performed. The valve was well-seated with no RVOT obstruction and no paravalvular leak (PVL). Total cross clamp for both procedures was 81 minutes and cardiopulmonary bypass (CPB) time was 117 minutes. Post-CPB echocardiography showed two well-seated prosthetic valves and trace pulmonary regurgitation (PR) from a trace-to-mild PVL. There was no evidence of TR or tricuspid valve stenosis (Video 1). The mean pressure gradient for the Perceval valve was 1 mmHg.
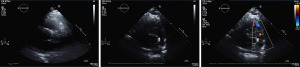
The patient was extubated on post-operative day 1 and did not require any blood products. Unfortunately, on post-operative day 3, the patient developed small bowel ischemia, which necessitated a small bowel resection. The patient was discharged home 14 days after his cardiac surgery. On 1-year follow-up, the patient is independent in his daily activities and does not elicit any cardiac symptoms. Repeat transthoracic echocardiography (TTE) at 18 months revealed two well-seated and well-functioning prosthetic valves, and no evidence of PVL for either valve. The peak and mean transvalvular pressure gradient for the Perceval valve in the pulmonic position were 16 and 10 mmHg, respectively (Figure 3).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and the accompanying image and the video. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
Sutureless valves represent an important option for surgeons in managing patients requiring complex or high-risk operations. For the first time, we report the successful use of a Perceval sutureless valve for PVR in a patient with significant carcinoid cardiac disease. Pre-operatively, despite appropriate medical therapy, the patient was in florid heart failure with moderate RV dysfunction in the setting of pulmonary stenosis and tricuspid regurgitation. The use of a Perceval sutureless valve facilitated an operation that required less suturing (ie. placing annular stitches for a sutured valve). At 18-month follow-up, the patient has a well functioning Perceval valve with normal transvalvular pressure gradients. At 24-month follow-up, the patient remains symptom-free and at his functional baseline.
Strengths and limitations
This case shows the safety and feasibility of using Perceval valve in the pulmonic position in patients presenting with a high-risk profile. The main limitation is that this is based on one patient, therefore more studies are needed.
Comparison with similar research
In addition to facilitating minimally invasive cardiac surgery, there is mounting evidence supporting the excellent clinical and hemodynamic outcomes associated with these prosthetic valves (6,7). Furthermore, an increasing number of studies have shown their safety and feasibility in patients with bicuspid valves, those with small annuli, the elderly, and in the setting of active infective endocarditis (4,8).
Explanation of findings
A Perceval valve was chosen for three main reasons: (I) to ensure a favorable trans-valvular pressure gradient, which was especially pertinent since the RVOT was extremely small; (II) to facilitate reducing the operative time by decreasing the need for sutures or a large patch to be sewn into the RVOT; and (III) allow for potential transcatheter interventions in the future.
Implications and actions needed
Although most of the cross-clamp time was dedicated to the TVR, shorter operative times and lesser sewing was imperative to reduce the risk for requiring blood product transfusions for this patient, who had severe carcinoid syndrome. Moreover, the Perceval valve has been shown to be favorable for transcatheter interventions for multiple reasons. Given this patient’s potential need for redo surgery, being able to employ transcatheter strategies in the future can be essential. Indeed, the medium-sized Perceval valve that was used in this case can accommodate larger sized percutaneous valves in the future. Finally, while more studies with larger case series and longer follow-up are needed, this case demonstrates that a Perceval bioprosthetic valve can be safely used for PVR in a high-risk patient.
Conclusions
Rapid deployment valves have helped shape the evolution of heart valve surgery over the past decade. In addition to mitigating the challenges of concomitant operations, these prostheses have facilitated performing complex procedures and/or operating on high-surgical risk patients. Herein, we present the case of a patient with severe carcinoid syndrome who underwent PVR using a Perceval rapid deployment valve. Although more extensive clinical studies are warranted, this case adds to the literature supporting the safety for deploying a Perceval valve in the pulmonic position in a high-surgical risk patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Aleksander Dokollari, Basel Ramlawi, and Gianluigi Bisleri) for the series “Sutureless Valves” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-34/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-34/coif). The series “Sutureless Valves” was commissioned by the editorial office without any funding or sponsorship. C.A. is a proctor for Corcym. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and the accompanying image and the video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kueri S, Berger T, Puiu PC, et al. The Hemodynamic Performance of the Perceval Sutureless Aortic Valve in a Propensity-Matched Comparison to the Carpentier-Edwards Perimount and Perimount Magna Ease Valves for Aortic Valve Replacement. Thorac Cardiovasc Surg 2023;71:542-9. [Crossref] [PubMed]
- Berretta P, Andreas M, Carrel TP, et al. Minimally invasive aortic valve replacement with sutureless and rapid deployment valves: a report from an international registry (Sutureless and Rapid Deployment International Registry)†. Eur J Cardiothorac Surg 2019;56:793-9. [Crossref] [PubMed]
- Chiariello GA, Bruno P, Villa E, et al. Aortic Valve Replacement in Elderly Patients With Small Aortic Annulus: Results With Three Different Bioprostheses. Innovations (Phila) 2019;14:27-36. [Crossref] [PubMed]
- Zubarevich A, Rad AA, Szczechowicz M, et al. Sutureless aortic valve replacement in high-risk patients with active infective endocarditis. J Thorac Dis 2022;14:3178-86. [Crossref] [PubMed]
- Pandit S, Annamaraju P, Bhusal K. Carcinoid Syndrome. Treasure Island (FL): StatPearls Publishing; February 13, 2023.
- Concistré G, Baghai M, Santarpino G, et al. Clinical and hemodynamic outcomes of the Perceval sutureless aortic valve from a real-world registry. Interdiscip Cardiovasc Thorac Surg 2023;36:ivad103. [Crossref] [PubMed]
- Kueri S, Berger T, Puiu PC, et al. The Hemodynamic Performance of the Perceval Sutureless Aortic Valve in a Propensity-Matched Comparison to the Carpentier-Edwards Perimount and Perimount Magna Ease Valves for Aortic Valve Replacement. Thorac Cardiovasc Surg 2023;71:542-9. [Crossref] [PubMed]
- Dokollari A, Ramlawi B, Torregrossa G, et al. Benefits and Pitfalls of the Perceval Sutureless Bioprosthesis. Front Cardiovasc Med 2021;8:789392. [Crossref] [PubMed]
Cite this article as: Fatehi Hassanabad A, Basha A, Noss C, Adams C. The novel use of Perceval valve for pulmonary valve replacement in carcinoid syndrome: a case report. J Vis Surg 2024;10:8.