Minimally invasive Ivor Lewis esophagectomy for esophageal cancer
Introduction
Treatment of esophageal cancer contains various surgical approaches and open surgical techniques still considered as the standard method (1). Ivor Lewis procedure involves laparotomy, thoracotomy followed by intrathoracic anastomosis. Transhiatal esophagectomy includes laparotomy and cervical anastomosis without thoracotomy. McKeown procedure involves a three-stage esophagectomy with cervical anastomosis. Each technique has potential advantage and disadvantage. Open Ivor Lewis and McKeown esophagectomy has a high morbidity and mortality. Pulmonary complications are the primary concern because of synchronous laparotomy and thoracotomy. Therefore, the transhiatal procedure is preferred in patients with reduced lung function. However, in the transhiatal procedure, mediastinal lymph node dissection is omitted, and the final stage could not be determined, resulted in a potential risk of recurrence.
Anastomotic leakage is another major problem in esophageal cancer surgery because of poor blood supply of gastric conduit. Anastomotic leakage in the cervical anastomosis is more frequent but less severe than intrathoracic anastomosis.
It is unclear that which procedure is optimal. The level of the anastomosis depends on the tumor location, underlying disease, and surgeon’s preference.
Minimally invasive esophagectomy (MIE) has been introduced since 1990’s and reduced pulmonary complications while technical challenging (2). MIE with cervical anastomosis is preferred due to technical feasibility in the early period. However, the cervical anastomosis is related to more leakage, stricture, and nerve injury compared with intrathoracic anastomosis (3).
With growing experience of minimally invasive surgery, intrathoracic anastomosis has been performed gradually for middle and lower esophageal cancer.
We demonstrated the technique and feasibility of high intrathoracic anastomosis under thoracoscopy.
Patient selection and workup
Between October 2010 and June 2015, Total 87 patients underwent subtotal esophagectomy for esophageal cancer by a single surgeon in Seoul St. Mary’s Hospital. MIE was performed in the 81 patients. The tumor location primarily indicated the level of the anastomosis. MIE McKweon procedure was performed in 10 patients for upper thoracic esophageal cancer. The indications of MIE Ivor Lewis operation (71 patients) were mid to lower esophageal cancer, non-T4 lesion and no history of laparotomy or thoracotomy.
The patients had to receive chest computed tomography (CT), positron emission tomography (PET) and endoscopic ultrasonography (EUS) to determine the clinical stage.
Echocardiography and pulmonary function test were performed routinely before surgery. If the advanced clinical stage without distant metastasis was diagnosed, neoadjuvant treatment was performed followed by surgery.
Pre-operative preparation
No special preparation is needed.
Equipment preference card
- Primary thoracoscopy set;
- Thoracoscopic instruments;
- Harmonic scalpel (Ethicon Endo-Surgery, Inc., Cincinnati, Ohio, USA);
- Endostapler (TriStapler; Covidien, Norwalk, CT, USA);
- EEA (DST EEA 28; Tyco, Healthcare, Norwalk, CT, USA).
Procedure
Abdominal procedure (Figure 1)

Patients placed in the lithotomy position after a double-lumen endotracheal tube placement. Five-ports utilized for gastric mobilization. A 10.5-mm port placed in the umbilicus for a 30° angle scope. Two 5-mm ports were placed bilaterally in the subcostal region at the mid-clavicular line, and 12-mm ports were placed between these two 5-mm ports on both sides. The assistant for the scope positioned between the patient's legs and the operator performed the procedure on the right aspect of the patient. The first assistant placed on the left side of the patient. Dissection of the omentum was carried out along the greater curvature of the stomach from the insertion of the right gastroepiploic artery using the scalpel (Ethicon Endo-Surgery, Inc.). The gastrosplenic ligament and short gastric vessels were divided, and the lesser omentum incised, then the dissection was performed to avoid the injury of the capsule of the pancreas. The left gastric artery and vein were identified and divided using a laparoscopic clip. The gastrohepatic ligament was then divided. The esophageal hiatus was identified and the lower esophageal part mobilized from the hiatus. To avoid stomach compression at the hiatus after the operation, we elect to widen the hiatus by a slight release and incision in the right crura. Pyloromyotomy was performed and surgical glue was applied. Partial gastric tubing procedure was performed using a linear stapler at distal two-third of the stomach alongside lesser curvature with a creation of new stomach pouch of 5 to 6 cm width. During the procedure, regional lymph nodes were dissected, and jejunostomy was not needed routinely (5).
Thoracic procedure (Figure 2)

After completion of the gastric procedure part, the patient’s position was changed to the left lateral decubitus, and 6-cm utility incisions with four ports were made on the right side of the chest. Utility incision was placed in the 6th intercostal space at the anterior axillary line with 6cm length. First port (10 mm sized) was positioned in the 8th intercostal space at the mid-axillary line for the thoracoscopy. The second port (10 mm sized) was placed in the 3rd intercostal space at the anterior axillary line. The third port (10 mm sized) was applied below the inferior angle of the scapula. The last port was implemented in the 6th intercostal space at the posterior axillary line (Figure 3). After selective single-lung ventilation, the azygos vein was divided using the Endostapler device (TriStapler; Covidien, Norwalk, CT). The mediastinal pleura were opened over the thoracic esophagus. Starting from middle esophageal dissection, the enbloc esophageal dissection was performed with adjacent loose tissue and lymph nodes from the heart and left mediastinal pleura. The esophagus was encircled using umbilical tape for esophageal lifting. The upper thoracic esophagus was dissected to the level of thoracic inlet. During the dissection at the tracheal level, extensive dissection was avoided to prevent tracheal injury or fistula creation between the trachea and gastric conduit. We identify the left recurrent laryngeal nerve while we retract the trachea anteriorly, and we dissect the lymph node meticulously using endo-scissors. The lower thoracic esophagus dissected, and the thoracic duct was ligated routinely, followed by mediastinal lymph nodes clearance.
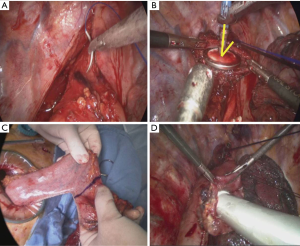
After mobilization of the intrathoracic esophagus, a manual purse-string suture of the muscular layer was placed at the highest level of the thoracic esophagus using 2–0 prolene® (Figure 4A).
Vertical esophagotomy was performed (3 to 4 centimeters) below the purse-string suture. The anvil of a 28-mm circular stapler was placed carefully in the proximal esophagus through the esophageal opening, and the purse-string suture was tied around the central rod (Figure 4B). Additionally, the esophagus was divided just below the tied purse string suture. The stomach pulled into the thorax through the hiatal opening, and the dissected esophagus and stomach were pulled out through the utility incision. The final gastric tubing procedure (>5 cm in width of gastric conduit) was carried out using a linear stapler, leaving a 4-cm opening at the top of gastric tubing for subsequent EEA body insertion (DST EEA 28; Tyco, Healthcare, Norwalk, CT; Figure 4C). The stomach graft was then returned to the thoracic cavity. The EEA body inserted into the stomach graft (Figure 4D), and the spike was penetrated the stomach wall. The anvil was approximated and attached to the EEA body, and the device was fired to create the anastomosis. After ensuring the internal mucosal integrity through the stomach opening, the opening was closed using a linear stapler, and after then we placed nasogastric tube. Frozen sectioning was checked proximal margins. The gastric tube was placed in the posterior mediastinum, and the whole length incised pleura closed with interrupted sutures for the prevention of gastric elongation or bulging into the pleural cavity (7). Finally, a single chest tube placed.
Role of team members
During the thoracoscopic phase, the operator has performed MIE Ivor Lewis operation on the patient’s left side. The first assistant was on the patient’s right side. The second assistant was next to the operator for the thoracoscopy.
The operator used utility incision for the grasper and 10 mm port in the 3rd intercostal space for the ultrasonic device.
The first assistant used 10 mm port below the subscapular area for endo-instrument and utility incision for the long curved suction device.
Postoperative management
The patients were referred to the general ward, and early ambulation started immediately. Patient’s education for deep breathing and active coughing exercise was delivered by specialized educators. Nasogastric tube aspiration was performed hourly for 4 hours and removed on a postoperative day 1 or 2 depend of amount of the drainage. Low molecular heparin was given to avoid deep vein thrombus.
Cephalosporin antibiotic was given for 2 days only in the absence of evident infection.
The chest tube was removed once the drainage is less than 200 mL/day.
Diet was started on a postoperative day 5 to 8 depending on the patient’s condition. The esophagography was not requested routinely.
Tips, tricks, and pitfalls
After middle thoracic esophageal dissection, the esophagus was circled with an umbilical tape or long silastic drain tube. The lifting of the esophagus allowed upper thoracic esophageal mobilization and node dissection safer and efficiently. We made a muscular purse-string suture using 2–0 prolene. The needle holder was placed through the 10 mm port in the 3rd intercostal space. Vertical esophagotomy performed below the purse string suture. In the MIE Ivor Lewis operation, the placement of the anvil in the proximal esophagus is troublesome. For that, 90 degrees between anvil and esophagus allowed easier placement of anvil in the proximal esophagus therefore we used the port in the 3rd intercostal space. During the approximation of anvil and EEA body, a gentle and tensionless approximation of stomach and the esophagus without adjacent soft tissue is needed. Pushing the body toward the apex of the chest generally makes tensionless anastomosis.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Muller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1900;77:845-57. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Rindani R, Martin CJ, Cox MR. Transhiatal versus Ivor-Lewis oesophagectomy: is there a difference? Aust N Z J Surg 1999;69:187-94. [Crossref] [PubMed]
- Jeon HW, Sung SW. Laparoscopic gastric tubing. Asvide 2016;3:436. Available online: http://www.asvide.com/articles/1208
- Lee JW, Sung SW, Park JK, et al. Laparoscopic gastric tube formation with pyloromyotomy for reconstruction in patients with esophageal cancer. Ann Surg Treat Res 2015;89:117-23. [Crossref] [PubMed]
- Jeon HW, Sung SW. VATS Ivor Lewis operation. Asvide 2016;3:437. Available online: http://www.asvide.com/articles/1209
- Jeon HW, Park JK, Song KY, et al. High Intrathoracic Anastomosis with Thoracoscopy Is Safe and Feasible for Treatment of Esophageal Squamous Cell Carcinoma. PLoS One 2016;11:e0152151. [Crossref] [PubMed]
Cite this article as: Jeon HW, Sung SW. Minimally invasive Ivor Lewis esophagectomy for esophageal cancer. J Vis Surg 2016;2:165.