Robotic-assisted sleeve lobectomy for right upper lobe combining with middle lobe resection of lung cancer
Case presentation
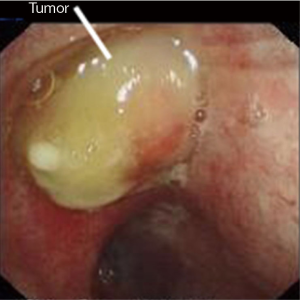
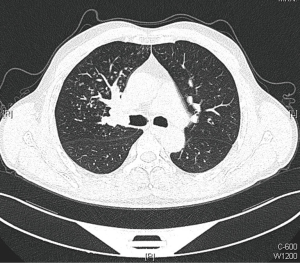
A 54-year-old male, smoker, who was admitted to our hospital due to repeated hemoptysis for 1 month. CT scan revealed a 4 cm × 3 cm × 3 cm mass located at the hilus of right upper lobe and the lobe bronchus was obstructed. Some sporadic small nodules were also discovered in the middle lobe with 3–5 mm in diameter (Figure 1). PET/CT scan was made to enhance the suspicion of malignance on both the lesions in the two lobes with high SUV. Squamous carcinoma was diagnosed by bronchoscope biopsy. The intermediate and middle lobe bronchus were not involved (Figure 2). The pulmonary artery and vein were free. The arterial blood gas analysis, pulmonary function test, and cardiac evaluation showed normal with no other comorbidities.
Surgical technique
General anesthesia with dual-lumen endotracheal intubation was given in the first place. The patient was then positioned in the left lateral decubitus position. We chose to apply a three-arm method using Da Vinci SI surgical system. The observing hole for camera port was made firstly at the 7th intercostal space in the midaxillary line. The other two ports were made at the 4th intercostal space in the anterioraxillary line for the right arm and the 7th intercostals space in the subscapular line for the left arm respectively. A 40-mm incision in the 6th intercostal space in the anterioraxillary line was made for assisted. The robotic surgical system was placed cephalad to the patient and one assistant on the ventral side. The video is shown in Figure 3.

The mass was confirmed in the hilus of the upper lobe and no other nodules were palpated in the pleura. We started from releasing the inferior pulmonary ligament to bring enough mobility followed by the dissection of the posterior wall of the hilum.
The subcarinal lymph nodes were carefully explored. We identified the vagus nerve top-down which run posterior to the superior vena cava and descends to the right main bronchus. Under the clear vision of three-dimensional high-definition camera, the operator dissected the vagus nerve. The branches contributed to pulmonary and esophageal plexuses were all clearly confirmed. The pulmonary branch was then carefully protected while the lymph nodes were removed. Systematic lymph node dissection was performed subsequently in the nerve sparing method.
Then mobilization and transection of the incomplete oblique fissure to identify the middle lobe arteries. The arteries were clipped by hem-o-lok and divided by scissor. The posterior ascending branch of the pulmonary artery was clipped in the same procedure. Next, the anterior trunk of the upper lobe was dissected and stapled, followed by the stapling of upper and middle lobe veins together trans the subscapular port after removing the left arm. Then the middle lobe bronchus was stapled via the assisted port. We encircled the right main and intermediate bronchi subsequently. The operator made markers at the pars cartilaginea with electrical hook and then transected with scissors respectively.
The lobes were removed in a glove for frozen pathology examination with the result of negative margins. The end-to-end bronchial anastomosis was performed between the main and intermediate bronchus subsequently. Firstly, the instrument in right arm was changed to needle holder. Then, two 3/0 prolene were utilized in proper order. We started continuous suture from the medial wall of the pars cartilaginea with the first prolene and the other end of the line was tightened by the assistant. Length of 5 cm to the needle end was retained to prepare for knotting, and then we brought in the second line to continue with the residual after finished the anterior wall. The two knots were made between the ends of the lines at two sides of anastomoses to ensure the continuity respectively. No leakage was detected and the estimated blood loss was less than 100 mL.
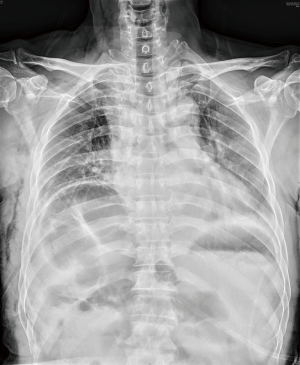
Postoperative chest X-rays showed no signs of atelectasis (Figure 4). The chest tube was removed on the 2nd postoperative day. The postoperative recovery was uneventful and the patient was discharged on the 5th postoperative day.
Comments
The application of robotic system in thoracic malignance has covered a history of decades since a primary lung cancer was treated by Melfi et al. (2) in 2002. This procedure has been proven to be safe and feasible in vast area benefited from the articulated movements and endo-wristed instruments (3-6).
The updated new generation robot system along with the development of instruments facilitate controlling of any operative step from the robot’s console, which further expand the indication of this technique. Sleeve resection has been an extension of the minimally invasive spectrum; however, lung cancer involving the bronchus via robotic procedure poses many challenges and used to be concerned as a contraindication (7). Ishikawa and colleagues described the first report on a robotic sleeve lobectomy in a human cadaver in 2006 (8).
We began performing VATS sleeve lobectomy from 2013 using two-port method. We designed the surgical plan for robotic sleeve lobectomy and completed our first left upper sleeve lobectomy using Da Vinci (Si) surgical system in January 2015 (9). Up to September 2016, over 25 cases of sleeve lobectomy were undertaken by robotic surgical approach. The preliminary results indicated the surgical procedure was safe and feasible in treating lung malignance.
The bronchial reconstruction with the anastomosis is concerned to be key steps. In our experience, the two ends of the bronchus would be brought together using running sutures with two 3–0 prolene lines. Due to the developments on robotic system, these technical advantages permit placement of precision microsutures in a deep operative field facilitating anastomosis without line twist. In case of sleeve lobectomy, we believe that utilizing the robot can not only increase the percentage of resection for lung cancer, but also extend the benefit to larger group patients including multiple lesions, bilobectomy and complex bronchial lesions.
The utility of this procedure also allows for a better lymph nodal clearance, however may contain a potential of increasing complications for nerve injury. Investigation in sparing of the pulmonary vagus nerve branches during minimal invasive lobectomy is lacking. Some studies have reviewed the pathophysiological processes associated with vagus nerve disorder. Eraslan et al. (10) found that loss of vagal innervation would impair both the ventilation and the oxygen uptake of lung in the dog. Krasna et al. (11) described most common types of vagus nerve injury in thoracic surgery and discussed the etiology and treatment. In our assessment, vagus nerve sparing may contribute to the development of postoperative pulmonary complications. In our experience, sparing of pulmonary vagus nerve branches during robotic surgery is feasible.
To make a deep investigation, long-term survival studies in patients with lung cancer should be carried out subsequently. The robotic surgery may not only contribute to improving safety and feasibility, but also hopefully to have a positive influence on overall outcomes.
Conclusions
Complicated sleeve lobectomy with nerve sparing is feasible in robotic thoracic surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Zhao Y, Chen H, Jiao W, et al. Robotic-assisted sleeve lobectomy for right upper lobe combining with middle lobe resection for lung cancer. Asvide 2016;3:488 Available online: http://www.asvide.com/articles/1263
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Wetscher G, et al. First experiences with the da Vinci operating robot in thoracic surgery. Eur J Cardiothorac Surg 2004;25:844-51. [Crossref] [PubMed]
- Iwata H. Minimally invasive pulmonary surgery for lung cancer, up to date. Gen Thorac Cardiovasc Surg 2013;61:449-54. [Crossref] [PubMed]
- Velez-Cubian FO, Ng EP, Fontaine JP, et al. Robotic-Assisted Videothoracoscopic Surgery of the Lung. Cancer Control 2015;22:314-25. [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Augustin F, Bodner J, Maier H, et al. Robotic-assisted minimally invasive vs. thoracoscopic lung lobectomy: comparison of perioperative results in a learning curve setting. Langenbecks Arch Surg 2013;398:895-901. [Crossref] [PubMed]
- Ishikawa N, Sun YS, Nifong LW, et al. Thoracoscopic robot-assisted bronchoplasty. Surg Endosc 2006;20:1782-3. [Crossref] [PubMed]
- Zhao Y, Jiao W, Ren X, et al. Left lower lobe sleeve lobectomy for lung cancer using the Da Vinci surgical system. J Cardiothorac Surg 2016;11:59. [Crossref] [PubMed]
- Eraslan S, Hardy JD. Differential division of hilar tissue: effects upon lung function in the dog. Dis Chest 1966;50:449-55. [Crossref] [PubMed]
- Krasna MJ, Forti G. Nerve injury: injury to the recurrent laryngeal, phrenic, vagus, long thoracic, and sympathetic nerves during thoracic surgery. Thorac Surg Clin 2006;16:267-75. vi. [Crossref] [PubMed]
Cite this article as: Zhao Y, Chen H, Qiu T, Xuan Y, Luo Y, Shen Y, Jiao W. Robotic-assisted sleeve lobectomy for right upper lobe combining with middle lobe resection of lung cancer. J Vis Surg 2016;2:178.