Nowadays open-chest surgery in the era of fast-track management
Introduction
Since Ferdinand Sauer Bruch, beginning the twentieth century, it was always difficult to perform a thoracic surgical procedure having at the same time a stable patient and a comfortable surgeon.
By those times of unsolved basic difficulties, incredibly complex solutions, like vacuum chamber were attempted.
Once those difficulties were overcome through the use of orotracheal intubation and positive pressure ventilation, the universally accepted approach for intrathoracic procedures was the posterolateral thoracotomy or “full thoracotomy”.
It meant to obtain a wide chest aperture by sectioning several chest wall muscles and performing a costo-transverse disarticulation when not cutting and/or removing a rib. Any procedure could be performed through this approach.
However, the severe pain derived from the extensive damage to the chest wall, brought about troublesome postoperative courses. High incidence of complications was the rule, as were prolonged lengths of stay and high costs. Almost all adverse events derived from pain-related hypoventilation and ineffective cough, not to mention late problems like chronic chest pain and even shoulder-girdle dysfunction.
Perioperative chest pain management
During the last two decades of the past century, literature was plenty of publications on the topic of postoperative chest pain with a surprising variation on the evaluation of its magnitude. They spanned from been considered a light event (1) to a central postoperative problem (2).
Most studies referred to measurement of pain severity, respiratory function compromise, identification of effective analgesic drugs and way of administration, trying to find a balance between invasiveness and effectiveness (2).
Few papers (3) referred to the multiple and complex mechanisms of postoperative pain when cutting several muscles and widely spreading ribs. Moreover, it was a rarity to find operative chest pain studies mentioning the availability of less painful and damaging techniques to perform open chest procedures, some of them very old techniques but still of preference even for complex procedures like lung transplantation (4).
Small, muscle-sparing thoracotomies
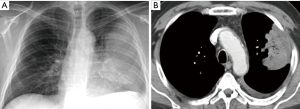
Two surgical innovations and technological advances changed the situation in a relative short period of time. From the end of eighties, the concept of muscle-sparing thoracotomy took relevance, once proved its technical feasibility and positive effect on immediate and late postoperative course (5,6). Full posterolateral thoracotomy as initially conceived, was only considered for those cases were the need for a wide thoracic aperture was preoperatively obvious (Figure 1).
But by the early nineties, the application of video-assisted techniques to thoracic surgical procedures was also a reality.
As usually happens with successful innovations, beginning present century two facts appeared: (I) widespread attempts to perform by video as much procedures as possible. Perhaps unconsciously, many surgeons were suspicious that open chest surgery was coming to an end and nobody wanted to remain in the past; (II) comparative studies between video-assisted thoracic surgery (VATS) and open chest performed procedures began to flourish in the literature. Some of them valuable, many others with severe limitations and arguable conclusions, not to mention the scarcity of randomized trials.
When analyzing those comparative studies, besides common limitations as retrospective analysis, not homogeneous series of patients and participation of different surgeons, two main drawbacks in many of them are worth to mention:
- The identification of open chest procedures simply as “thoracotomy”, with no additional information on what type of chest aperture had been performed;
- Limitation of the study to the immediate postoperative course.
Contributions of those studies to general knowledge of the problem and to the design of recommendations have been variable and somehow confusing.
Superiority of VATS was clearly demonstrated in several studies (7,8), others reported only subtle differences favoring VATS (9) or still others found the advantages of VATS limited to special situations like surgery on the elderly (10).
Although Hartwig and D’Amico (11) make clear their view that VATS lobectomy should be considered the gold standard for “early-stage” lung cancer, they also consider VATS a “reasonable option” for lung cancer management, implicitly recognizing the existence of other options. Moreover, they correctly highlight an important point: some surgeons may not be trained and experience enough to perform VATS lobectomy independently.
Finally, it is difficult to understand Nagahiro’s finding (12) of a mean drop of just 5% in forced expiratory volume in one second after VATS lobectomy, in comparison with the known mean drop of 20% after thoracotomy lobectomy. If such a huge difference become the general experience, it would be almost impossible to find a place for open chest lobectomy, not to say of patients with limited lung function.
Only recently, it began to be recognized that making a valid comparative study between VATS and open procedures is a more complex task than initially thought, particularly when considering some aspects like pain evaluation. As we all know, many factors have their influence in patient’s perception of pain, including the expectations on the advantages of VATS he/her may have overestimated, based on the information received either from the surgeon or through the media.
Chest retractors evolution and minimally-access open chest surgery
Since the beginning of twentieth century, many attempts have been made in chest retractor’s design to obtain the best possible access to intrathoracic structures (13).
Although far from perfect, the Finochietto retractor designed in 1941 became almost universally adopted.
The fact that the advent of small-access muscle-sparing thoracotomy, with its special needs of chest retraction, did not elicit the design of truly innovative instruments is not easy to explain. Perhaps the appearance of VATS distracted the attention of surgeons and industry from the issue. Only the use of two crossed Finochiettos became popular.
Nevertheless, limitations of Finochietto retractor became even more evident when used in cases of short skin incisions preserved muscles and limited rib spreading. Only intrathoracic structures located just in front of that sort of “tubular” surgical field were really at reach of vision and instruments (Figure 2).
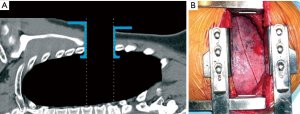
The unique way to improve that field was additional rib spreading which, besides being against the central concept of small access thoracotomy, put the patient at risk of rib fracture and intercostals neurovascular damage. In any case, the surgical field remained “tubular”.
With all these observations in mind and after a long time of laboratory and designers work, we were able to develop a new-concept chest retractor that only share with Finochietto retractor the toothed rack as a mechanism for rib spreading.
It’s articulated main arms, multiple “rib friendly” main blades and accessory blades to retract uncut muscles, allow to obtain a “conical”, not “tubular” surgical field through a four to five inches skin incision, two inches rib spreading and no costo-transverse disarticulation (Navarro Thoracic Retracto, Delacroix-Chevallier, Paris, France). After a very short experience it can be set up in a few minutes (Figure 3).
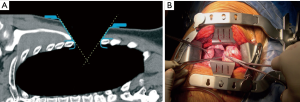
Although some special instruments improve the retractor’s performance, a safe and comfortable procedure can be performed with standard thoracic surgical instruments and a mild postoperative course usually follows.
In using this retractor, surgeons must be respectful of three technical aspects:
- Chest wall muscles around the thoracotomy site must be dissected-free to allow their displacement;
- Muscle sectioning must be kept to a minimum. Usually one third of latissimus dorsi muscle in case of posterolateral thoracotomy and no muscle cutting at all in case of antero-axillary thoracotomy;
- After making an intercostal muscle cutting much longer than skin incision, ribs are spread no more than two inches (Figures 4,5).


Preoperative choosing surgical approach and postoperative course
Although we regularly perform VATS lobectomy for clinical early-stage lung cancer, and obviously use video thoracoscopy for diagnostic procedures and surgical treatment of pneumothorax, hyperhidrosis, trauma and so on, it is our policy to rely on minimally invasive open chest surgery for higher stages of lung cancer, chest wall surgery, and mediastinal tumors of significant size, when sternotomy is not indicated.
Information given to patients before consent always includes the characteristics of VATS and open chest surgery, as well as our reasons to select one approach or the other. When VATS is chosen, deliberately we do not advise them about the possibility of conversion to open in case of the approach has not been adequate. We understand choosing approach as a preoperative decision and keep the conversion option for the management of intraoperative crisis when necessary.
In our experience, a regular patient for a minimally-invasive open chest lobectomy or segmentectomy is admitted the same day of surgery. After a 2 hours long procedure, patient spent 24 hours at intensive care unit (ICU) followed by 2 or 3 additional days in the ward. Chest drains removal and discharge usually takes place on postoperative day 3 or 4.
Pain management, which in the past included a thoracic epidural catheter for no less than three days with addition of intravenous analgesics if necessary, is now exceptionally used only in those cases requiring a full posterolateral thoracotomy.
Our present analgesia schema includes: Intraoperative bupivacaine intercostals nerves block and intravenous narcotics, changed on day three to oral tramadol plus ketorolac which are maintained at home usually less than a week after discharge.
Unfortunately we were not able to perform the necessary randomized trial comparing identical procedures performed by VATS vs. minimally-invasive open chest, neither we are aware of such a study appearing at least in English literature. A recent randomized trial (16) shows some results favoring VATS regarding postoperative pain and quality of life, although it refers only to an early-stage lung cancer group of patients and there are numerous limitations recognized by the authors in their conclusions.
Our unpublished observational findings in a series of one hundred consecutive patients who had a minimally-invasive open chest lobectomy for stages I to IIIA lung cancer are resumed in Table 1.
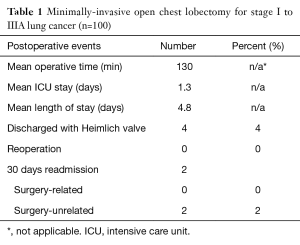
Full table
As can be seen in our experience, fast-track management is possible too in open chest surgery performed through a minimally-invasive muscle-sparing thoracotomy using adequate instrumentation. Same day admission and non-invasive chest pain management should also be considered first election.
Although powerful, randomized trials are still lacking, some studies suggest that immediate postoperative course and moreover, late postoperative results may be no so different and even worse in using VATS, provided a valid comparison is made (17), as it is its relationship with long term survival (18). Recent publications suggest technical ways to improve this crucial aspect of surgical lung cancer management (19,20).
In future comparative studies, to the description of unquestionable advantages of VATS, a detailed description of the thoracotomy used must be added. Moreover, study design should exclude from the comparison full thoracotomy patients or those open chest procedures were merely an uncut preservation of serratus mayor was made.
Conclusions
Even after the marked changes derived from the advent of VATS with its unquestionable advantages for performance of many procedures, absolute and relative indications remain for open chest surgery, although it rarely means the full posterolateral thoracotomy of the past.
Nowadays open chest surgery should be understood as opening the chest in a minimally-invasive way.
Maximal sparing of chest wall muscles, minimal rib spreading and use of adequate instrumentation should be cornerstones to keep in mind when considering opening the chest. Obviously, these concepts must be honestly included in the preoperative information given to the patient.
If this requisites were accomplished, either VATS or open chest surgery, once correctly indicated for every individual patient and surgical problem will became what everybody wish: an effective and cost-effective way to perform thoracic surgical procedures ending in satisfactory an identically good immediate and late results.
Moreover, in borderline cases, approach decision will be easier to take and “conversion to open” will become what it should be, a last resort for better management of intraoperative crisis.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Ricardo Navarro discloses a financial relationship with Delacroix-Chevalier, Paris, France. And other author has no conflicts of interest to declare.
References
- Salzer GM, Klingler P, Klingler A, et al. Pain treatment after thoracotomy: is it a special problem? Ann Thorac Surg 1997;63:1411-4. [Crossref] [PubMed]
- Soto RG, Fu ES. Acute pain management for patients undergoing thoracotomy. Ann Thorac Surg 2003;75:1349-57. [Crossref] [PubMed]
- Alister JR, Puttappa A, Harney D. Post-Thoracotomy Pain Syndrome. In: Guerreiro Cardoso PF. editor. Topics in Thoracic Surgery. Rijeka: InTech, 2012:81-90.
- Pochettino A, Bavaria JE. Anterior axillary muscle-sparing thoracotomy for lung transplantation. Ann Thorac Surg 1997;64:1846-8. [Crossref] [PubMed]
- Baker NH. Muscle sparing thoracotomy. Ann Thorac Surg 1988;46:369. [Crossref] [PubMed]
- Uzzaman MM, Robb JD, Mhandu PC, et al. A meta-analysis comparing muscle-sparing and posterolateral thoracotomy. Ann Thorac Surg 2014;97:1093-102. [Crossref] [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg 2008;85:231-5; discussion 235-6. [Crossref] [PubMed]
- Hartwig MG, D'Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg 2010;89:S2098-101. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Bonfils-Roberts EA. The rib spreader: a chapter in the history of thoracic surgery. Chest 1972;61:469-74. [Crossref] [PubMed]
- Navarro R, Benavidez R. Minimally-invasive postero-lateral thoracotomy, aimed to show chest aperture and retractor’s setting. Intrathoracic procedure itself can be performed at surgeon’s preference. Asvide 2016;3:500. Available online: http://www.asvide.com/articles/1275
- Navarro R, Benavidez R. Minimally-invasive antero-axillary thoracotomy, aimed to show chest aperture and retractor’setting. Intrathoracic procedure itself can be performed at surgeon’s preference. Asvide 2016;3:501. Available online: http://www.asvide.com/articles/1276
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Medbery RL, Gillespie TW, Liu Y, et al. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2016;11:222-33. [Crossref] [PubMed]
- Smeltzer MP, Faris N, Yu X, et al. Missed Intrapulmonary Lymph Node Metastasis and Survival After Resection of Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:448-53. [Crossref] [PubMed]
- Liu C, Pu Q, Guo C, et al. Non-grasping en bloc mediastinal lymph node dissection for video-assisted thoracoscopic lung cancer surgery. BMC Surg 2015;15:38. [Crossref] [PubMed]
- Lee PC, Kamel M, Nasar A, et al. Lobectomy for Non-Small Cell Lung Cancer by Video-Assisted Thoracic Surgery: Effects of Cumulative Institutional Experience on Adequacy of Lymphadenectomy. Ann Thorac Surg 2016;101:1116-22. [Crossref] [PubMed]
Cite this article as: Navarro R, Benavidez R. Nowadays open-chest surgery in the era of fast-track management. J Vis Surg 2017;3:1.