Tips and tricks to decrease the duration of operation in robotic surgery for lung cancer
Introduction
Minimally invasive surgery (MIS) continues to grow in popularity in virtually all fields of surgery, including in the treatment of lung cancer. Thoracoscopic lobectomy has demonstrated improved perioperative outcomes, decreased pain, and similar long-term survival compared to open thoracotomy for patients with early-stage non-small cell lung cancers (1-3). Robotic-assisted surgery is an evolution of minimally invasive thoracic surgery that is becoming increasingly common (3,4). Its most visible benefits include a dramatic advance in visualization with magnified, high-definition, three-dimensional imaging coupled with upgraded instrument maneuverability, building upon what have often been cited as critical weaknesses of video-assisted thoracoscopic surgery (VATS): limited, two-dimensional visualization along with restricted maneuverability (1,5,6). Moreover, robotic thoracic surgery has been demonstrated to reduce perioperative complications and hospital length of stay with similar effectiveness to open thoracotomy; direct comparisons to VATS, while limited, suggest similar efficacy (6-9).
However, one of the most significant criticisms of robotic surgery, in addition and related to cost, is longer operative time (5,9,10). This is frequently associated with the reportedly steep learning curve that comes with robotic surgery as a consequence of its distinct instrumentation and technique (5,9,10). These are valid considerations, as longer operative durations have been shown to independently increase potential for infectious complications and length of hospital stay (11). Compounding this, cost estimations peg an additional minute of operating room time between $22 and $133 in the United States (12). In addition, long operative times can make it more difficult from practicality and safety standpoints to teach robotic surgery to residents at academic medical centers, and surgeons not confident in their robotic operative technique cannot adequately or responsibly serve as mentors for their trainees (13,14). Nevertheless, these hurdles are neither inherent nor inevitable drawbacks. Rather, they can be minimized not only with increased familiarity with the robotic system but also the adoption of efficient techniques—both preoperatively and intraoperatively—married to an intimate understanding of the relevant anatomy. The objective of this paper is to expound upon such tips and tricks to safely decrease the duration of operation in robotic surgery for lung cancer.
Operative technique tips and tricks
Approach each operation with a well-defined, well-communicated systematic plan
Preoperative planning is pivotal to improving intraoperative efficiency. Since 2010, the year of our first robotic lobectomy, we have developed and refined systematic procedures that optimize patient outcomes while enabling an environment conducive to teaching residents (14). These robotic lobectomy techniques have been arranged as a series of major chronologic steps, defined for each of the five different types of lobectomy, as seen in Table 1 (14). These steps are identified repeatedly before and during operations, allowing every team member—nurses, anesthesiologists, students, and residents—to be aware of both what is happening and what will happen next. In doing so, our multidisciplinary team can prepare for and, ideally, anticipate next moves, facilitating communication and smoothening transitions between different stages of an operation. For instance, circulating and scrub nurses in our operating rooms have become familiar with when the best time is to procure and ready a stapler for the pulmonary artery, minimizing wasted time between requesting a stapler and deploying it. Thus, it is essential to embrace the obvious and develop and reinforce systematic plans that are shared with and understood by the operative team.
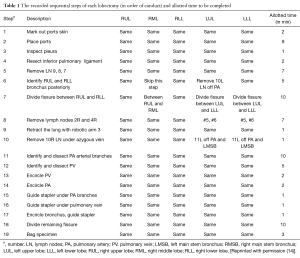
Full table
Determine the desired duration of each step in the plan
Dovetailing with the centrality of a systematic plan is the importance of outlining how long each sequential step should take. We have done just that: Table 1 delineates both the steps to our procedures as well as their respective desired durations (14). These assigned durations were derived from our previous experience as well as videos of other surgeons’ robotic operations (14). When adhering to this methodology, operative time can be efficiently but safely minimized and should be under two hours (14). The practice of assessing and analyzing the duration of each step provides twofold benefits. First, by breaking down the steps that are requiring the most time relative to desired duration, areas for improvement can be distinguished and addressed. Second, by keeping track of each operation’s progression in real time, surgeons can quickly and quantitatively identify when an operation is likely to take longer than anticipated, and adjust accordingly.
Value stream preoperative protocols
Operating room preincision time is not technically included in the duration of operation and is often left woefully inefficient as a result. However, time in the operating room—whether it is preoperative, intraoperative, or postoperative—is inextricably linked to cost and outcomes. Furthermore, a systematic, optimized approach characterized by value streaming—whereby every action is evaluated as value-added or not—with defined roles can produce dramatic time and cost savings; our protocol decreased preoperative time from 64 to 37 minutes, on average (15).
With this protocol, we have optimized patient positioning with foam pads and tape to ensure adequate anesthesia access without sacrificing surgical maneuverability (Figure 1). In so doing, we have phased out the use of axillary rolls, arm boards, and beanbags. Intraoperative central catheter use has been virtually eliminated (75% of cases to 0% of cases) by establishing, in collaboration with anesthesia, criteria to only place one after we are unable to acquire two peripheral intravenous access sites (15). Similarly, intraoperative arterial catheter use has been dramatically reduced (93% of cases to 4% of cases) by largely restricting them to patients who have had coronary artery stenting in the past six months, a recent stroke with unresolved ipsilateral carotid artery stenosis, or post-induction hemodynamic instability (15). Epidural catheter use has also been curtailed (84% of cases to 3% of cases) by transforming pain management to include pre-induction acetaminophen (850 mg) and gabapentin (900 mg) by mouth, intraoperative subpleural paravertebral bupivacaine hydrochloride injections (0.25% with epinephrine), and postoperative acetaminophen, oxycodone, and lidocaine patches (15). Finally, our Foley catheter use has gone from essentially reflexive to selective (99% of cases to 11% of cases) by largely restricting them to patients that regularly have two or more episodes of nocturia (15).
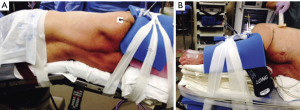
Standardize endotracheal tube placement
Endotracheal tube selection and placement can be optimized for time and outcomes with a defined protocol (15). We first place a single-lumen endotracheal tube in patients who have smoked within the last three months (due to concern for secretions), who have a history of abnormal bronchoscopy, or who have a computed tomography scan indicating what could be an abnormal bronchoscopy. This is done for both diagnostic and therapeutic reasons. Then, a double-lumen endotracheal tube is placed, and is our standard approach for robotic lobectomy. Anesthesiologists who are not experienced with them can struggle with this step; however, we have used a simple and reproducible technique to facilitate rapid and correct placement of the double-lumen tube. The following describes placement of a left-sided double lumen tube (a right-sided tube is generally reserved for a left pneumonectomy or sleeve resection). The patient’s trachea is intubated with the tube. A pediatric bronchoscope is inserted into the bronchial lumen and advanced into the left main stem bronchus. The endotracheal tube is then advanced over the pediatric bronchoscope, which effectively acts a guide. The bronchoscope is then inserted into the tracheal lumen to assess the bronchial cuff’s location and ensure proper depth of the tube. The tube is then secured with tape.
Optimize port positioning
Positioning of the patient, the operative team, the robotic ports, and the robot itself is a critical yet underappreciated key to efficient operations. Ports are carefully and methodically inserted to maximize maneuverability of robotic instruments, optimize access to the critical structures, and avoid collisions; of note, we attempt to use smaller ports where possible to minimize postoperative pain (Figure 2) (16,17). For the da Vinci Si system, we use two 8 mm ports (left and right robotic arm ports), a 12 mm port (camera), and one 5 mm port (fourth robotic arm port); for the Xi system, all the ports are 8 mm ports. We also utilize a 12 mm assistant port that can be used for stapling and exchange of items such as rolled-up sponges and vessel loops. The assistant port is also important in case sudden or catastrophic bleeding occurs. The following is a description of port placement for a right-sided resection.
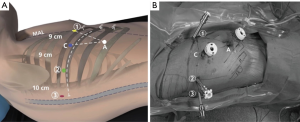
All ports are marked before making an incision, although slight changes to these locations are often necessary once the intrathoracic anatomy is visualized. The general guideline is that the ports are located in the seventh (upper or middle lobectomy) or eighth (lower lobectomy) intercostal space. The fourth robotic arm is located 2–3 cm from the spine, the left robotic arm port is located 10 cm away from that port, the camera port is located 9 cm from the left robotic arm port, and the right robotic arm is located 9 cm away from the camera port (Figure 2). We insert the camera port first and perform an intercostal nerve block from the exterior using the spine as a guide. We then place the fourth robotic arm port, positioning it two ribs beneath the oblique fissure. The camera is then inserted through the fourth robotic arm port and the other two ports are subsequently inserted under direct vision. The assistant port is a 12 mm port and is inserted just superior to the diaphragmatic fibers—and hence as anteroinferior in the chest as possible—while being triangulated between the camera and right robotic arm ports. This isosceles triangle positioning maintains excellent robotic arm maneuverability while securing adequate space for the bedside assistant. For the Si system, the robot itself is subsequently steered at a fifteen-degree angle over the patient’s shoulder and then docked. For the Xi system, the robot can approach the operating room table perpendicular to the patient, after which the beam is rotated to the proper position. We utilize a zero-degree camera instead of a thirty-degree one due to its decreased torque, which reduces the chances of intercostal nerve injury (16).
Adapt efficient intraoperative processes
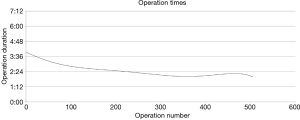
It is difficult to quantify how particular technical details can help decrease operating time. However, certain concepts and practices have proved helpful to us as we have refined our technique and safely increased the speed of our lobectomies over time (Figure 3) (14).
We perform our mediastinal lymph node dissection first—this ensures a thorough dissection and, especially in the case of level 7 lymph nodes, helps facilitate isolation of hilar structures. We believe that the use of carbon dioxide insufflation saves time by decreasing the size of the lung parenchyma (improving visualization) while also decreasing bleeding secondary to increased intrathoracic pressure. Naturally, optimal retraction of the lung is critical. As in VATS lobectomy, removing lymph nodes prior to encircling structures leads not only to improved lymphadenectomy but helps facilitate the safe isolation and division of vessels and bronchi. We retract vascular structures gently with rubber vessel loops and use a curved tip stapler when encircling them, processes which we believe help facilitate what are generally the most intimidating steps of the lobectomy. Removal of the resected lung is protocolized into steps to avoid clumsy and dangerous handling of specimen removal: (I) place the specimen in the fourth robotic arm and maneuver the arm up and away; (II) position the bag away from hilar structures; (III) pull down on the tip of the bag as it is being deployed to ensure that it opens in the correct direction; (IV) drop the specimen into the bag with the fourth robotic arm; (V) hold the back of the bag with the fourth robotic arm; (VI) use the left and right robotic arms to push the specimen into the bag; and (VII) let go of the bag with the fourth robotic arm and have the assistant close the bag.
Although we have listed the conventional order of steps during robotic lobectomy, it is important to be flexible and recognize the fact that individualized patient anatomy (variations include incomplete versus complete fissure) can dictate a rearrangement of steps to make the operation faster. For instance, isolating and dividing the bronchus first during a right upper lobectomy can make the rest of the operation simpler. Dividing the fissure first, which is often saved for last in VATS lobectomy, can also be helpful in certain situations. We generally do not reinsufflate the lung to “test” it after the bronchus to be resected is clamped, as we believe it is an unnecessary step in most cases.
Discussion
MIS for the treatment of lung cancer continues to grow in popularity for its superior perioperative outcomes as well as comparable long-term survival relative to open thoracotomy for early-stage non-small cell lung cancers (1-3). Within the domain of MIS, robotic surgery represents a frameshift by ameliorating many of the weaknesses that have bedeviled VATS. For this reason, we strongly believe that robotic thoracic surgery represents the future of lung cancer surgery. As it continues to evolve and surgeons grow more experienced with it, operative time and outcomes from robotic surgery will continue to improve, as they have been shown to already (18,19).
In addition to the benefits of familiarity, however, there are a multitude of strategies to reduce operating room and operative times while maintaining or even improving patient safety and outcomes. This is vital, as every minute of wasted time in the operating room is costly and potentially hazardous. We have presented six tips and tricks to this end: (I) develop and communicate a systematic plan of action; (II) determine and track the desired time for each sequential step; (III) utilize value streaming preoperative protocols; (IV) standardize endotracheal tube placement; (V) optimize port positioning; and (VI) adapt efficient intraoperative processes.
The suggestions in this paper draw on our many years of accumulated experience and fine-tuned techniques, which we have published in the literature. Hence, a strength of this paper is that the tips and tricks described have been extensively practiced and optimized, ensuring that they do not sacrifice patient safety or outcomes. The main limitation of this paper is that, by virtue of basing itself on the recorded experience of a single institution, the paper’s generalizability cannot be proven. Furthermore, the process of optimizing the efficiency of our operating room and robotic surgery is a continuous one; therefore, it is impossible to individually quantify the benefit of any of these particular interventions. However, we were careful to craft our descriptions so that they can be standardized. We look forward to widespread adoption and subsequent study of these strategies—and other innovations—to further evaluate their respective benefits while identifying ways to improve upon them. This will enable us to realize the full potential of robotic surgery for the treatment of lung cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: O Ramadan: none; B Wei: Medtronic—speaker; RJ Cerfolio: Intuitive Surgical—proctor, speaker, lecturer; Ethicon—speaker, teacher; Community Health Services—consultant; KCL—consultant; Bovie—consultant; C-SATS—consultant.
References
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Rocco G, Internullo E, Cassivi SD, et al. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin 2008;18:235-47. [Crossref] [PubMed]
- Morgan JA, Ginsburg ME, Sonett JR, et al. Advanced thoracoscopic procedures are facilitated by computer-aided robotic technology. Eur J Cardiothorac Surg 2003;23:883-7; discussion 887. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Velez-Cubian FO, Ng EP, Fontaine JP, et al. Robotic-Assisted Videothoracoscopic Surgery of the Lung. Cancer Control 2015;22:314-25. [PubMed]
- Nakamura H. Systematic review of published studies on safety and efficacy of thoracoscopic and robot-assisted lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2014;20:93-8. [Crossref] [PubMed]
- Rinieri P, Peillon C, Salaün M, et al. Perioperative outcomes of video- and robot-assisted segmentectomies. Asian Cardiovasc Thorac Ann 2016;24:145-51. [Crossref] [PubMed]
- Bryant AS, Rudemiller K, Cerfolio RJ. The 30- versus 90-day operative mortality after pulmonary resection. Ann Thorac Surg 2010;89:1717-22; discussion 1722-3.
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Procter LD, Davenport DL, Bernard AC, et al. General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg 2010;210:60-5.e1-2.
- Macario A. What does one minute of operating room time cost? J Clin Anesth 2010;22:233-6. [Crossref] [PubMed]
- Maertens H, Aggarwal R, Desender L, et al. Development of a PROficiency-Based StePwise Endovascular Curricular Training (PROSPECT) Program. J Surg Educ 2016;73:51-60. [Crossref] [PubMed]
- Cerfolio RJ, Cichos KH, Wei B, et al. Robotic lobectomy can be taught while maintaining quality patient outcomes. J Thorac Cardiovasc Surg 2016;152:991-7. [Crossref] [PubMed]
- Cerfolio RJ, Steenwyk BL, Watson C, et al. Decreasing the Preincision Time for Pulmonary Lobectomy: The Process of Lean and Value Stream Mapping. Ann Thorac Surg 2016;101:1110-5. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 1095-6. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Davini F, et al. Robotic lobectomy for lung cancer: evolution in technique and technology. Eur J Cardiothorac Surg 2014;46:626-30; discussion 630-1. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Davini F, et al. VATS-based approach for robotic lobectomy. Thorac Surg Clin 2014;24:143-9. [Crossref] [PubMed]
Cite this article as: Ramadan OI, Cerfolio RJ, Wei B. Tips and tricks to decrease the duration of operation in robotic surgery for lung cancer. J Vis Surg 2017;3:11.


