Video-assisted thoracoscopic bronchoplasty
Patient selection
- Endobronchial tumours;
- Direct invasion of bronchial origin by tumour or metastatic nodes.
Operative procedure
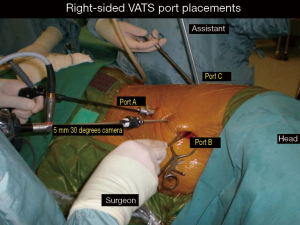
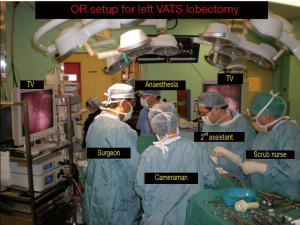
The video-assisted thoracoscopic surgery (VATS) lobectomy technique used is a totally endoscopic procedure performed by directly watching a TV monitor. The following non rib spreading ports are placed, a 5 mm 30 degrees camera placed over the major fissure at 5th intercostal space, a 10 mm retraction port placed at the 6th/7th intercostal space posterior and inferior to the scapula tip, a 10 mm working port at the 6th/7th intercostal space anterior axillary line and a 2 cm non rib spreading utility incision at the 3rd intercostal space (Figure 1). The lobectomy is done through an anterior approach where the surgeon and camera assistant stands in front of the patient with the 2nd assistant and scrub nurse on the opposite side (Figure 2). The port placements and the position of the team remains the same for all lobes of both lungs and the camera stays in the same position throughout the procedure. All lobar vessels are individually dissected and divided. The endostaplers for the vessels, bronchus and fissures are introduced through the lower most working port. All patients undergo complete radical mediastinal lymphadenectomy.
Left-sided double lumen endotracheal tube is routinely placed for all VATS lobectomy. However a right side double lumen tube is placed whenever possible for left sided sleeve resections. In left sided sleeve resections, a left sided double lumen tube tends to splint and immobilizes the left main stem bronchus making approximating and sewing of the two bronchial ends without tension difficult. Also in long segment left bronchial sleeve resection especially when involving the left main stem bronchus the tube and cuff comes in the operative field. This makes approximating and sewing of the two bronchial ends without tension difficult.
All bronchial margins are subjected to intraoperative frozen section analysis to ensure clear tumour margins before performing the anastomosis. The anastomosis should when possible be done last to prevent injury or disruption to the anastomosis. The anastomosis is done tension free with mucosa to mucosa approximation without approximation by using interrupted or continuous absorbable monofilament sutures (polydioxanone PDS 40 OR 50) with all knots placed on the outside. Sewing is routinely done with a 5 mm endoscopic needle holder. Monofilament suture allows for smooth passage of sutures through the bronchus and facilitates in the sliding and tying of knots. The monofilament sutures allows for smooth placement and sliding of knots.
Types of bronchoplasties
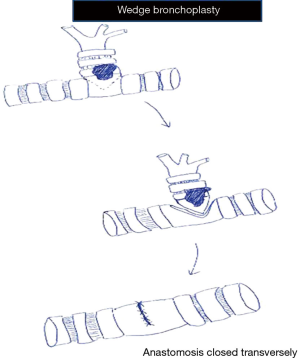
In wedge bronchoplasty, in addition to the lobar bronchus a wedge of the adjacent main airway is included for clear margins. The anastomosis is closed transversely to prevent narrowing of the airway (Figures 3,5). This is especially to be noted when closing the right lower lobe bronchial stump to prevent narrowing of the middle lobe bronchus (Figure 6). Studies have shown that wedge bronchoplasties are a good oncological equivalent to sleeve bronchoplasties especially for small tumours with limited invasion of the bronchus (3,4). Besides better preservation of vascular supply they are easier to perform by VATS allowing surgeons embarking on bronchoplasty to perfect their sewing and tying before doing full sleeve lobectomies.


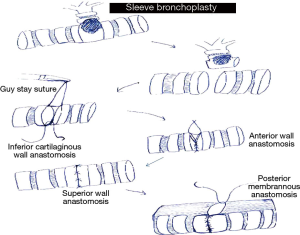
In sleeve lobectomies release procedures like division of the inferior ligament are routinely done to relieve tension on the anastomosis (5). Exposure and sewing is enhanced by taping and retracting the main pulmonary artery if necessary. In long segment resections where there is a wide distance between the two bronchial ends a stay suture at the distal or between the proximal and distal anastomosis, held by the assistant helps to approximate the two ends and relieve tension when approximating the ends. Sewing should always start on the inferior part of the cartilaginous part of the anastomosis then moving up the anterior wall and then lastly the superior wall. Size matching of incongruous proximal and distal ends is achieved by performing the membranous part of the anastomosis last as it allows for size matching and prevents tearing of the thin walled part of the bronchus (6). This is best done by rotating the patient anteriorly towards the surgeon in a 45–60 degrees semi prone position where the falling away of the lung under gravity allows for an easy anastomosis. Intraoperatively, integrity of anastomosis is checked bronchoscopically and by insufflating air under water at 30 cm of pressure. Whenever concomitant pulmonary angioplasty is needed it is performed by obtaining proximal and distal vascular control by applying endoscopic vascular clamps. The vascular repair is done with 60 polypropylene sutures. None of the anastomosis is buttressed with flap or any vascularized tissue (Figures 6-8).


For right VATS middle lobectomy anastomosis of the intermediate to lower lobe bronchi is hindered by the overriding lower lobe pulmonary artery. This is overcome by taping the pulmonary artery and retracting it and the lung anteriorly. With the pulmonary artery out of the way the anastomosis is done easily (Figure 9).

For left VATS upper sleeve lobectomy the cartilaginous part of the anastomosis is completed after the pulmonary artery is retracted superiorly. The pulmonary artery is then taped and retracted anteriorly together with the lung. This gives good exposure to the membranous part for the anastomosis to be completed (Figure 10).

A VATS sleeve lobectomy is easier to perform as exposure for suturing of the two divided ends of the bronchi is easier after division of the vessels and removal of the affected lobe. However these advantages are lost in VATS lung sparing main stem sleeve resections as the overlying undivided vasculature and airways of the lobes limit exposure for mobilization and sewing. Difficulty of sewing is further enhanced on the left side by the aorta arching over the anastomosis. Maneuvers to improve exposure include taping of the left main pulmonary artery and mobilizing it proximally and distally after division of the major fissures. Similar mobilization of the left lower lobe and upper lobe bronchi are done under the taped and retracted pulmonary artery. The left lung and left main pulmonary artery are retracted anteriorly for optimal exposure of anastomosis of the left main stem bronchus with the upper and lower lobe bronchial origins. Retraction of the aortic arch through the posterior 5 mm port also enhances exposure during anastomosis.
Figure 11 shows a lung sparing left main stem sleeve resection in a 43-year-old female non-smoker with a 1 month history of fever and cough. CAT scan thorax showed an endobronchial tumor 2 cm from carina at junction of left main stem bronchus and upper and lower lobe bronchial origins (Figure 3). There was also evidence of scarring of the posterior segment of the lower lobe from recurrent chronic infections from distal obstruction. Bronchoscopy showed a completely occluded left main stem bronchus by tumour. Biopsy confirmed a low grade mucoepidermoid carcinoma. PET CT showed low FDG avid tumour with no evidence of metastases. After resolution of the lung infection patient underwent a VATS sleeve main stem bronchus resection of the tumour. Due to the proximity of the tumour to the carina a right sided double lumen endotracheal tube (DLET) was used. The VATS was done through an anterior approach as described above. Despite lung isolation with DLET the left lung remained hyperinflated due to air trapping from distal obstruction from the tumour. The major fissures between upper and lower lobes were divided with staplers. The left main pulmonary artery was taped and mobilized proximally and distally in the major fissure. Similar mobilization of the left lower lobe and upper lobe bronchi was done under the taped and retracted pulmonary artery. The inferior ligament was released to allow for tension free anastomosis. The left lung and left main pulmonary artery were then retracted anteriorly for optimal exposure of the left main stem bronchus, the upper and lower lobe bronchial origins.

Once adequate mobilization of the bronchi was obtained a bronchotomy over the tumour site was done. Mucus distal to the airway due to obstruction was aspirated as it allowed for better deflation of the lung enhancing exposure. The tumour was noted to arise focally from the posterior wall of the bronchus at junction of upper and lower lobe bronchial origins. The tumour was excised. One centimeter proximal and distal bronchial margins were additionally excised and send for frozen section analysis. Once the bronchial margins were confirmed to be negative for malignancy the proximal and distal bronchi were reanastomosed. The anterior and cartilaginous bronchial wall was re-approximated with continuous 40 polydioxane (PDS) suture. The PDS is an ideal suture for bronchial anastomosis as it is absorbable and monofilament allowing easy passage and approximation of the anastomosis. The posterior membranous wall of the anastomosis was completed with interrupted sutures as this allows for better size matching of the proximal and distal anastomosis. The bronchial anastomosis was then checked by intraoperative bronchoscopy and for air leak by underwater insufflation under 30 cm water pressure. Operative time was 150 mins. Patient was discharged well on 3rd postoperative day. Final histology was a 2 cm low grade mucoepidermoid carcinoma with clear margins. All lymph nodes sampled were negative for malignancy.
Results
Between Dec 2006 and December 2009, 21 out of 231 (9.1%) of VATS lobectomy cases underwent VATS bronchoplasty.12 were females and 9 males. Mean age was 64.9 years (range, 47–83 years) Indications for bronchoplasty were endobronchial tumours in 3, direct invasion of lobar bronchus by tumour 6 and metastatic lymph nodes in 12. Seventeen were elective bronchoplasties and 4 were incidental cases where bronchial invasion was detected only at time of surgery. Mean hospital stay was 5.2 days (range, 3–8 days). Mean duration of surgery was 287 mins (range, 135–540 mins). Nine had simple or wedge bronchoplasty, 8 sleeve bronchoplasties and 4 complex or extended bronchoplasties. Right upper lobe sleeve lobectomies are the commonest being the easiest to perform. There was no operative mortality. 19 cases were for non-small cell lung carcinoma (NSCLC) 1 for carcinoid and 1 for colonic metastasis. Among the NSCLC, 5 were in stage Ib, 5 in IIa, 2 in IIb and 7 in IIIa. Mean follow up was 26.2 months (range, 6–32 months). To date one patient developed bronchopleural fistula 8 weeks after surgery. He developed a small 5 mm fistula at superior edge of the left upper lobe stump after undergoing a simple bronchoplasty with pericardial and chestwall resection. The previous prolene mesh over the chestwall defect was removed and a latissimus dorsi myocutaneous flap was used to close the fistula and the chestwall through the defect. To date no anastomotic strictures or local tumour recurrence were noted. One patient had brain relapse at 18 months (2).
Preference card
- 5 mm endoscopic needle holder and 5 mm Maryland dissector;
- 40 or 50 PDS absorbable monofilament suture for bronchial anastomosis;
- Aesculap endoscopic vascular bulldogs (curve or straight);
- Endoscopic knot pusher;
- 30 degrees 5 mm camera lens.
Tips and pitfall
- Experience in bronchoplasty needs proficiency in endoscoping sewing and tying. This is acquired by routine sewing whenever possible in standard VATS lobectomy;
- Surgeon should be proficient in both intra and extracorporeal sewing and tying to suit the circumstances of the clinical situation;
- The surgeon should gain gradual experience in performing simple and wedge bronchoplasties before embarking on sleeve and more complex bronchoplasties;
- All margins must be subjected to intraoperative pathological analysis before performing anastomosis to prevent unnecessary revisions;
- Tension free anastomosis is mandatory especially in long segment resections and is ensured by release maneuvers and by a stay suture at the distal anastomosis or between the proximal and distal anastomosis. This is held by the assistant to approximate the two ends and relieve tension when the knots are tied;
- In wedge anastomosis bronchus is closed transversely to prevent narrowing of the main airway;
- General preference is to do cartilaginous anastomosis before membranous to prevent tearing of thin wall membranous wall and for easy correction of size discrepancy between the two bronchial ends;
- A combination of interrupted or continuous sutures for the anastomosis can be used depending on the distance and size discrepancy between the proximal and distal anastomosis;
- Pulmonary angioplasty is performed by using endoscopic bulldogs to prevent clamps coming in way of sewing;
- All anastomosis should be routinely checked by intraoperative bronchoscopy for integrity. This allows for immediate remedial anastomotic corrections if necessary.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Agasthian T. Right upper lobe video-assisted thoracoscopic surgery (VATS) wedge bronchoplasty. Asvide 2017;4:030. Available online: http://www.asvide.com/articles/1336
- Agasthian T. Right video-assisted thoracoscopic surgery (VATS) upper lobe sleeve lobectomy. Asvide 2017;4:031. Available online: http://www.asvide.com/articles/1337
- Park SY, Lee HS, Jang HJ, et al. Wedge bronchoplastic lobectomy for non-small cell lung cancer as an alternative to sleeve lobectomy. J Thorac Cardiovasc Surg 2012;143:825-831.e3. [Crossref] [PubMed]
- Agasthian T. Initial experience with video-assisted thoracoscopic bronchoplasty. Eur J Cardiothorac Surg 2013;44:616-23. [Crossref] [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- Chakaramakkil MJ, Jim LY, Soon JL, et al. Continuous absorbable suture technique for tracheobronchial sleeve resections. Asian Cardiovasc Thorac Ann 2011;19:44-7. [Crossref] [PubMed]
- Agasthian T. Right video-assisted thoracoscopic surgery (VATS) lower lobe sleeve lobectomy. Asvide 2017;4:032. Available online: http://www.asvide.com/articles/1338
- Agasthian T. Left video-assisted thoracoscopic surgery (VATS) lower lobe sleeve lobectomy. Asvide 2017;4:033. Available online: http://www.asvide.com/articles/1339
- Agasthian T. Right video-assisted thoracoscopic surgery (VATS) middle lobe sleeve lobectomy for endobronchial carcinoid tumour. Asvide 2017;4:034. Available online: http://www.asvide.com/articles/1340
- Agasthian T. Left video-assisted thoracoscopic surgery (VATS) upper lobe sleeve lobectomy/angioplasty. Asvide 2017;4:035. Available online: http://www.asvide.com/articles/1341
- Agasthian T. Left video-assisted thoracoscopic surgery (VATS) main stem lung sparing sleeve resection. Asvide 2017;4:036. Available online: http://www.asvide.com/articles/1342
Cite this article as: Agasthian T. Video-assisted thoracoscopic bronchoplasty. J Vis Surg 2017;3:12.