Robotic lung resections: video-assisted thoracic surgery based approach
Introduction
Video-assisted thoracic surgery (VATS) lobectomy started in the first half of 1990s, and two decades were necessary for VATS lobectomy before becoming a mature procedure in the early stage lung cancer. Despite long debates and negative attitude of experienced thoracic surgeons, VATS lobectomy established its place in the field of thoracic surgery.
Most of its difficulties were due to long learning curve of handling hilar dissection in a closed chest cavity. Long learning curve may be due to the lack of binocular visual system and wristed instrumentation. In addition, a camera controlled by another surgeon may be one of the reasons of the long learning curve. Robotics enabled rapid adoption in minimally invasive approaches for pelvic, cardiac and colorectal surgery, where vision and maneuverability are limited with open and laparoscopic approaches. In the past 10 years, robotic surgery has been adopted by thoracic surgeons unequivocally, and proved to have at least similar or better outcomes compared to VATS or open surgery, in terms of lower rate of complications, less blood loss, shorter hospital stay, less pain, and faster return to normal quality of life (1-4). Fast learning curve, provided by high definition three-dimensional camera, enhanced surgical maneuverability and precise surgery, has developed robotic lung surgery in the past 5 years. We have completed 5 years of active practice in the field of thoracic surgery with more than 250 cases. The aim of this study is to share our experience with VATS based approach.
In order to benefit from abovementioned superiorities, a surgical technique to dock is needed. This technique should provide the followings: Easy, uncomplicated and a platform to obtain the best capabilities of the robotic arms. Then, there remains a discussion regarding the optimum approach for the port placement. The debate is mostly on total port approach versus VATS based approach. VATS based approach is a “robotic-assisted approach” which is supported mostly by a table surgeon and an access thoracotomy. Each technique has its advantages and disadvantages. This paper aims to discuss the VATS based approach.
Technical details in VATS based approach
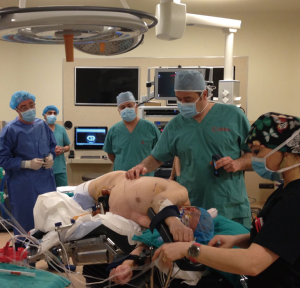
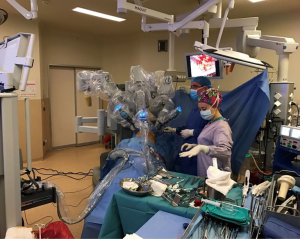
The patient is ready after the confirmation of single-lung ventilation with the fiberoptic bronchoscope, and the lateral decubitus position is given (Figure 1). The table is tilted either anteriorly or posteriorly, or kept in neutral position depending on the type of resection to be performed. The hilum of the lobe or the segment which was aimed to be resected is the target. Three ports were opened while trying to keep 10 cm between each port and 10–15 cm from the target. In VATS based approach the camera is in the middle and right and left arms 10 cm or more away lateral and medial to the camera. VATS triangle is usually kept as in a diamond shape, in which the target is the apex and the camera is the base. The camera is placed in the middle port. The technique we described here is used for Da Vinci SI Systems.
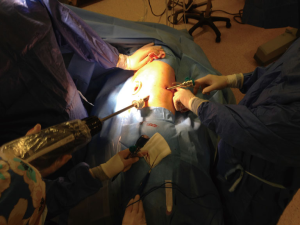
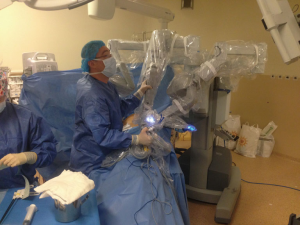
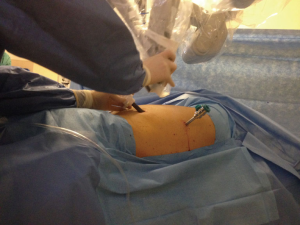
We firstly prefer opening the camera port on the 8th midaxillary intercostal space. While opening other ports, a 30 degree up camera is used. The second port is opened at the 8th or 9th intercostal space approximately 10 cm away from the camera port, and located close to the paravertebral sulcus (Figure 2). The anterior port is selected to be in a higher location like 6th or 7th intercostal space anterior to the camera port (Figure 3). All ports are opened following preemptive intercostal Marcaine injection. In the upper lobectomies and segmentectomies of the upper lobes, the access port is opened at the posterior intercostal space in the 10th or 11th intercostal space, after the docking has been completed as the 4th incision. In this case, anterior port is only for the right robotic arm. The robot is docked from the posterior by keeping 30 degrees between the vertebral column of the patient and transverse axis of the cart (Figure 4). Keeping the robotic camera in the up position, all the ports and instruments were placed safely. The service port was opened at the 10th–11th intercostal space at the posterior part of the thoracic wall to be used for suctioning, retracting, and taking the specimens out in upper lobectomies and segmentectomies of the upper lobe. This port is covered with Alexis soft tissue skin retractor (Applied Medical, Rancho Santa Margarita, CA, USA). Right after docking has been completed, intercostal nerve block was performed immediately with the aim of having preemptive analgesia. The rest of the operation was performed with the camera in the down position.
Lower lobectomies and lower lobe segmentectomies
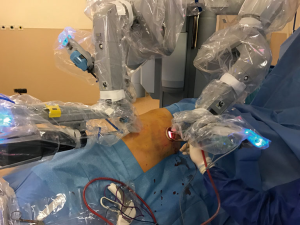
All three ports are opened in a similar fashion as described above, except for that the anterior port is opened as the access port, and it was covered with ALEXIS soft tissue skin retractor (Applied Medical, Rancho Santa Margarita, CA, USA) (Figure 5). By this way, the anterior arm (left in left sided resections and right in right sided resections) could be de-docked and re-docked easily whenever a stapler is introduced to provide the best environment for the table surgeon. Aspiration or retraction could be maintained by sharing this access port with the arm of the robot (Figure 6).
Standard flow of the operation in the VATS based approach
Maryland or curved bipolar forceps for the right arm and prograsper for the left arm were used, and the positions were changed as needed. First thing we do is to perform intercostal nerve block at 5–6 levels before starting the operation. During the resection and lymph node dissection, all the resected materials were extracted through the service port which was covered with ALEXIS soft tissue skin retractor (Applied Medical, Rancho Santa Margarita, CA, USA), and the main tissue containing the tumor was extracted using a plastic endobag. Individual dissection and division of the hilar structures were performed with endoscopic staplers introduced through the service port unless a specific introduction was needed, from either the right or left robotic arm ports. The incomplete fissures were divided either with a stapler introduced by the assistant surgeon through one of the ports, or with bipolar cauterization. Segmentectomies have been similarly performed (5,6).
Advantages
This approach is ideal for novices experienced in the VATS surgery. First of all, for an experienced VATS surgeon, converting to VATS is easy without any need for a thoracotomy. It carries similarities with the VATS technique and allows a VATS surgeon to feel comfortable in case of need to convert to a VATS operation instead of a thoracotomy. Especially when the surgeon wants to feel the tissue resistance during dissection, dissection of a particular vessel may be provided by the VATS based approach. The second most important benefit is to allow palpation of the nodules to be wedged which is almost always impossible in the total port approach. The third important advantage is as two arms are used, this operation is cheaper than the total port approach. There is also hypothetical advantage of less pain compared to four ports approach due to lesser incisions. The anterior incision has always has a potential to convert it to an appropriate thoracotomy is the last advantage.
Since we have a long standing experience in the VATS anatomical lung resections and thymectomy operations, we preferred to start with the VATS based approach. Now, after establishment of an experience level we can also perform total port approach. We speculate that total port approach necessitates a level of expertise in the field of robotic surgery which could be provided with duration of VATS based approach.
Disadvantages
If the table surgeon is a standard surgeon and developed capabilities, including de-docking and re-docking and vessel stapling, VATS based approach is extremely safe. However, if the table surgeon is novice, if it is a kind of duty to be done by a shift system, VATS based approach may be cumbersome. By using one arm less, the console surgeon sometimes may feel incapability at making appropriate retraction of the lung, particularly in station 7 dissection, if the assistance could not provide enough support. Four arm VATS based approach may be a solution to this discomfort. In VATS based approach, the most important disadvantage is CO2 insufflation could not be provided due to large access incision opened to room air. The posterior access incision used for upper lobe resections and segmentectomies of the upper lobe could not be converted to a useful thoracotomy to overcome a major problem from the upper lobe vessels. This is because the level of thoracotomy would be low in this situation. When there is a need for an open conversion, another thoracotomy from anterior is recommended.
Discussion
Briefly, the specific robotic techniques utilized are as follows: completely portal four arm technique (1); a completely portal three-arm technique with 5 cm extraction incision (7); and a three- or four-arm technique with a 3 cm to 4 cm non-rib spreading utility incision (3). VATS based approach is consistent with the 3–4 arm technique with a non-rib spreading utility incision. In VATS based approach a utility incision is created to help in retraction, suction and dissection by the table surgeon. This access port is also used to extract the large specimen out. In this surgery, since there is a communication with the intrathoracic cavity and the operating room environment, the benefits of CO2 insufflation could not be used. The second platform is the completely portal robotic lobectomy (CPRL) which allows entire procedure through the ports. Thus, definitely CO2 insufflation is allowed and helpful in this situation. The specimen is extracted by enlarging the most inferior port.
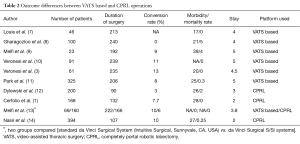
The comparison of both techniques could be seen in Table 1. Both types of platforms have similar perioperative outcomes. The outcomes are compared in Table 2. According to authors, outcomes are not different in both approaches. Routine use could be recommended based on the surgeon’s and center’s preferences.

Full table
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Weksler B, Tavares J, Newhook TE, et al. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg Endosc 2012;26:261-6. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. [Crossref] [PubMed]
- Demir A, Ayalp K, Ozkan B, et al. Robotic and video-assisted thoracic surgery lung segmentectomy for malignant and benign lesions. Interact Cardiovasc Thorac Surg 2015;20:304-9. [Crossref] [PubMed]
- Toker A, Ayalp K, Uyumaz E, et al. Robotic lung segmentectomy for malignant and benign lesions. J Thorac Dis 2014;6:937-42. [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Melfi FM, Ambrogi MC, Lucchi M, et al. Video robotic lobectomy. Multimed Man Cardiothorac Surg 2005;2005:mmcts.2004.000448.
- Veronesi G, Agoglia BG, Melfi F, et al. Experience with robotic lobectomy for lung cancer. Innovations (Phila) 2011;6:355-60. [Crossref] [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg 2012;1:24-6. [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Davini F, et al. Robotic lobectomy for lung cancer: evolution in technique and technology. Eur J Cardiothorac Surg 2014;46:626-30; discussion 630-1. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [Crossref] [PubMed]
Cite this article as: Toker A, Kaba E, Ayalp K, Özyurtkan MO. Robotic lung resections: video-assisted thoracic surgery based approach. J Vis Surg 2017;3:15.