
Training in minimally invasive thoracic surgery on 3D-model: back to the future of education
Introduction
Minimally invasive surgery (MIS) is the standard of care for early-stage lung cancer, offering benefits such as faster recovery, shorter hospital stays, smaller scars, and reduced postoperative pain (1). However, these procedures require advanced motor skills and a thorough understanding of anatomical relationships (2). Recent advancements, including specialized surgical instruments, and energy devices, have significantly improved surgical capabilities (3).
Traditional surgical training follows an observation-to-hands-on approach, but endoscopic procedures demand unique manual skills due to limited access and reliance on two-dimensional visualization. The steep learning curve of video-assisted thoracic surgery (VATS) and robotic-assisted thoracic surgery (RATS) underscores the need for simulation-based education, particularly for young surgeons (4,5). Simulation provides a controlled, risk-free environment where trainees develop technical proficiency before performing surgery on actual patients (6).
Virtual reality (VR) simulators are increasingly common, and VR simulators for thoracoscopic and robotic surgery, such LapSIM (Surgical Science, Goteborg, Sweden) and dV-Trainer Mimic Technologies (Surgical Science), are widely used in Europe and the U.S. (7). While these technologies help surgeons grasp theoretical principles and basic movements, real analog models remain crucial for refining complex skills and providing tactile feedback that closely resembles reality (8).
Simulation centers worldwide use basic lung models made from inert materials, wet labs, and cadaver training. However, the latter two methods pose limitations due to cost, availability, and ethical concerns. Advances in 3D printing and biomimetic materials now enable the creation of highly realistic models that interact with surgical energy sources, representing a promising alternative for training (9).
Training with realistic models enhances education by bridging theoretical knowledge from virtual platforms with hands-on practice (10-12). The University of Pisa’s EndoCAS Center is a leading multidisciplinary simulation facility for MIS.
The University of Pisa’s EndoCAS Center is a multidisciplinary simulation facility dedicated to enhancing surgical education through innovation and technology. Over the past 2 years, EndoCAS has developed a structured, cost-effective, and reproducible training program that integrates theoretical instruction, VR simulation, and hands-on practice with a custom-built 3D-printed thoracic cage and commercial high-fidelity lung models. This multimodal approach exposes thoracic surgery residents to a progressive and comprehensive learning experience—from conceptual understanding to manual performance (13,14).
This paper aims to illustrate the design and implementation of an integrated training pathway combining VR and physical simulation for thoracic surgery residents, with a specific focus on the structured simulation of lung lobectomy procedures. The purpose was therefore to develop a learning method for residents and young surgeons that could serve as a foundational model for a more advanced project currently under development, with the ultimate goal of enabling a more objective comparison between this model and other existing learning systems.
Methods
EndoCAS: a multidisciplinary approach to surgical simulation
Established in 2005, EndoCAS is managed by an interdisciplinary team of medical professionals, engineers, and computer scientists aiming to enhance surgical training through cutting-edge simulation technologies. EndoCAS scientists collaborate with leading surgeons, including key opinion leaders, across multiple surgical specialties such as plastic, general, vascular, and thoracic surgery, performing traditional and mini-invasive procedures. Thoracic surgery residents at EndoCAS engage in structured training sessions designed to develop their fundamental skills in MIS. Through exposure to high-fidelity virtual simulators such as LapSim and dVTrainer, trainees familiarize themselves with laparoscopic instruments and practice essential procedures including endoscopic suturing, knot-tying, and vessel coagulation. The performance of each trainee is objectively evaluated and recorded, ensuring a data-driven approach to competency assessment.
All participants in the program were thoracic surgery residents from University of Pisa. The training cohort included five senior residents (two PGY-5 and three PGY-4) and four junior residents (one PGY-3 and three PGY-1). Each trainee underwent a structured simulation curriculum that began with VR training on the LapSim simulator. This phase included 20 exercises: 10 basic skills modules focused on foundational techniques (e.g., camera navigation, hand-eye coordination, and instrument handling) and 10 advanced tasks involving more complex procedures (e.g., knot tying, cutting, and simulated surgical dissection). The exercises were designed with increasing levels of difficulty and evaluated through an automatic performance score (0–100) based on multiple criteria including execution time, movement efficiency, precision, error rate, and tremor control. An exercise was considered passed only if the score was ≥90%, and trainees could not proceed to the next task unless the previous one was successfully completed. Only after passing all 20 exercises were trainees admitted to the next training phase involving physical simulation on the 3D anatomical chest model.
3D-model development and lobectomy simulator
To enhance the realism of surgical simulation, a dedicated thoracic cage model was created using a 3D printer, based on computed tomography (CT) segmentations of a structurally normal patient’s thorax. Developed in collaboration with EndoCAS biomedical engineers, the full-size model replicates all bony structures of a hemithorax, including the sternum, vertebrae, and ribs. Notably, three ribs (at the IV and V intercostal spaces, commonly used for uniportal-VATS lung surgeries) were designed with mobile articulations to mimic joint-like movement. The model is covered with a tissue-like material to simulate human skin and anchored to the operating table using a wooden frame, ensuring structural stability during the simulation (Video 1).
The simulator consists of two key components: the lung module and the thoracic framework. The lung module (Right Lobectomy Model and Left Lobectomy Model, Fasotec Co., Ltd., Chiba, Japan) accurately replicates lung tissue properties, and broncho-vascular structures, allowing residents to practice dissection, ligation, and resection techniques in a controlled environment.
The development of the simulator follows a structured workflow integrating engineering principles with surgical requirements to ensure anatomical accuracy, biomechanical realism, and effective training. A CT scan from a real patient was used to generate a raw model of the thoracic cavity, which was refined using Blender (version 4.2.2). Key design features include replaceable ribs to account for damage during procedures, interchangeable rib types for simulating pathological conditions, structural rigidity to mimic chest biomechanics, and a synthetic skin layer made of Dragon SkinTM silicone (Smooth-On, Inc., Macungie, PA, USA) to prevent visibility into the simulator while allowing practice of the initial incision. 3D printing was selected as the preferred manufacturing method, utilizing a Stratasys 3D printer (Ortus F170) for ABS components and a Bambu Lab X1C 3D printer for thermoplastic polyurethane (TPU) flexible joints, ensuring optimal stiffness to replicate real rib resistance. The final assembly includes secure housing for the lung module, mounted onto a wooden base to maintain stability and realism during training (Figure 1). This setup effectively replicates the conditions of a real surgical procedure, enhancing residents’ proficiency in performing lobectomies with precision while reducing patient risk (15).
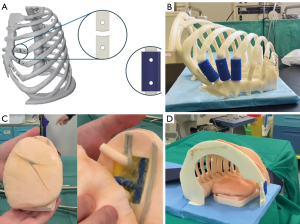
Simulation description
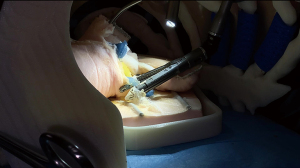
As reported in Video 2, a lower right lung lobectomy was performed in a simulated setting, with a senior resident as the first operator and a junior resident as the second. An experienced thoracic surgeon guided them, providing structured feedback on technical skills, surgical strategy, and particularly on critical steps such as stapler application angles, tissue handling, and exposure optimization (Figure 2).
To replicate real-world surgical conditions, the simulation was conducted in the same operating room used for clinical procedures. The 3D anatomical model was securely mounted to the operating bed using a support frame to ensure immobility. Standard uniportal-VATS instrumentation was employed, including endoscopic staplers, energy devices, graspers, and a 10-mm, 30-degree thoracoscopic camera, with real-time visualization on a monitor. The 3D anatomical chest model was positioned in the left lateral decubitus, simulating a real patient. The rib articulations allowed simulated table flexion. Uniportal-VATS access was established at the fifth intercostal space, using a soft tissue retractor (Alexis, Applied Medical, Rancho Santa Margarita, CA, USA) before inserting the thoracoscopic camera and surgical instruments (Scanlan International, Inc., Saint Paul, MN, USA).
The simulation began with an exploration of the pleural cavity, lung parenchyma, and mediastinum.
Residents were instructed on optimizing camera positioning and lung retraction to enhance exposure. During lung mobilization, the supervising surgeon emphasized the importance of avoiding excessive traction and maintaining gentle but effective manipulation to identify the pulmonary ligament and hilar structures. Dissection of the pulmonary ligament and lymph node station 9 was performed using an energy device, during which trainees were taught to avoid thermal spread and ensure adequate tension.
Identification of the lower lobe vein was guided, and the surgeon highlighted strategies for preserving vascular landmarks until proper exposure was achieved. During fissure dissection, attention was given to creating adequate space for stapler insertion without injuring adjacent parenchyma. The use of a rubber loop was demonstrated as a technique to safely guide stapler positioning. Correct identification of segmental arteries and sparing of the middle lobe artery were stressed as key steps in anatomical preservation.
For vascular division, residents were trained on selecting the correct stapler cartridge (gold/blue for arteries and white for veins), and special emphasis was placed on choosing the optimal insertion angle through the uniportal access to avoid stapler clashes or parenchymal injury. Proper planning of stapler trajectory and minimizing intra-thoracic adjustments were identified as critical challenges and teaching moments.
During bronchial isolation and division, the supervising surgeon provided targeted coaching on exposing the bronchus without compressing it, aligning the stapler jaws correctly, and confirming complete sealing. The resected specimen was then extracted using a retrieval bag through the 3 cm access.
A technical debrief followed the procedure. The most frequent errors identified included incorrect grasping of lung tissue, excessive traction, poor hand coordination, and difficulty in positioning instruments through a single port. Stapler handling was the most challenging aspect, especially regarding trajectory planning and achieving optimal positioning without damaging the parenchyma (Figure 3).
Lymphadenectomy of stations 7, 8, 9, 2R, and 4R was performed, with didactic emphasis on avoiding injury to the right recurrent laryngeal nerve. At the end of the procedure, before placing the chest drain, the tutor created discontinuities of varying size and location to teach the residents how to manage potential air leaks or bleeding. Aerostasis was simulated by arbitrarily creating discontinuities in the lung parenchyma, representing air leaks of varying size. Trainees were instructed to manage these defects by applying either single interrupted sutures (for small leaks) or a running suture (for larger ones), mimicking real intraoperative scenarios. Hemostasis was similarly simulated by designating specific sites and degrees of “bleeding”, challenging residents to choose the most suitable intervention—such as the use of energy devices, manual compression, or topical agents. Since actual hemostatic patches are costly, we employed materials with similar texture and size to represent them. This allowed residents to practice proper handling and timely application techniques, which was the main educational goal of this part of the simulation. Drain placement was demonstrated and secured with a U-suture. Simulated were reviewed with feedback on effective use of hemostatic agents and endoscopic suturing techniques.
The session concluded with a performance review involving the supervising surgeon and residents, during which the recorded video was analyzed to highlight areas for improvement and reinforce good practices.
The anatomical model presented specific challenges, including limited flexibility of lung tissue, increased resistance during stapler or endoloop advancement, and absence of realistic bleeding feedback in case of vessel injury. These characteristics demanded refined tissue handling and strategic planning, thereby enhancing the educational value of the simulation.
Discussion
The integration of physical anatomical models into surgical training represents a crucial step in bridging the gap between virtual simulations and real-life operative procedures (8). While VR-based training provides an essential preliminary learning phase, allowing residents to familiarize themselves with procedural steps, anatomical landmarks, and surgical decision-making in a risk-free environment, it lacks tactile feedback—a fundamental aspect of surgical proficiency. The transition from digital to physical models is therefore necessary to develop fine motor skills, depth perception, and realistic instrument handling, which are crucial in minimally invasive thoracic surgery (13). Returning to analog, hands-on models after initial exposure to virtual simulations ensure that residents internalize the spatial relationships of anatomical structures and refine their ability to apply controlled force, manipulate delicate tissues, and perform precise resections, particularly in uniportal-VATS lobectomies where limited access demands a high degree of technical precision. The lung model used in our training pathway is fabricated using biologically derived materials, offering a consistency and mechanical response that closely mimics real pulmonary tissue in vivo, including interaction with surgical instruments and energy devices. Additionally, the model offers a highly accurate anatomical representation, especially of delicate structures such as lymph nodes, which are critical for thoracic oncologic procedures and are rarely replicated with such precision in existing simulators.
The integration of simulation into thoracic surgery training has demonstrated significant benefits in skill acquisition, confidence building, and error reduction among trainees (16). Previous studies have compared different simulation methods, emphasizing the advantages and limitations of VR versus physical models. Jensen et al. in 2014 conducted a randomized controlled trial comparing VR-based simulation to black-box simulation in VATS lobectomy training (8). Their findings showed that while VR improved procedural planning and cognitive load management, trainees who trained on physical simulators performed significantly faster and demonstrated greater proficiency in instrument handling during subsequent live procedures. Similarly, Nashaat et al. in 2019 reviewed various lobectomy simulators and concluded that physical models provide a more comprehensive learning experience by integrating technical skills with realistic anatomical structures (6). Our experience aligns with these findings. The incorporation of a customized 3D-printed thoracic model with high-realistic lung models provided residents with a unique opportunity to translate their theoretical knowledge and VR-acquired psychomotor skills into a hands-on surgical scenario, enhancing their spatial awareness and tissue manipulation skills.
Furthermore, physical simulation models foster teamwork, as they require coordination among the primary operator, camera assistant, and a third assistant responsible for tissue traction, mirroring real operating room dynamics and reinforcing the importance of communication in complex procedures. Unlike VR, which is often a solo experience, hands-on training encourages interactive learning and role rotation, allowing multiple trainees, including surgical nurses, to gain exposure and experience in the same session. Additionally, performing procedures in a physical setting with real surgical instruments provides a more authentic experience, helping residents familiarize themselves with operating room ergonomics, equipment handling, and troubleshooting challenges in real-time.
Despite the advantages of high-fidelity phantoms, certain limitations remain. Unlike tissue in vivo or cadaveric models, synthetic models cannot replicate the dynamic properties of human tissue, such as vascular responses or lung expansion during ventilation. Moreover, the reduced tissue elasticity of the models, even if it is challenging as it provides less mobility than the real one, it may result in less delicate manipulation of the parenchyma in-vivo by the residents.
Future improvements in material technology and bioengineered models may further enhance their realism and effectiveness in surgical training (13). Investment in improving material fidelity, with high-realistic haptic feedback, and developing cost-effective production methods will further enhance their utility, making physical simulation a core component of thoracic surgery training (2).
This study presents some important limitations. First, there is a lack of structured, objective assessment of the training outcomes. Although the hybrid simulation program was pilot-tested on a cohort of 10 thoracic surgery residents, no formal pre- and post-training evaluations or validated scoring systems were applied in this preliminary phase. As a result, it is not currently possible to quantify the impact of the training on technical skill acquisition or surgical performance. Moreover, the study design did not include a control group, limiting any comparative analysis with other training modalities. Second, a detailed cost analysis of the program was not performed. This is primarily because the majority of the resources used were already available within the EndoCAS simulation center and the University of Pisa, including tutors, VR simulators, and 3D printing materials. The only direct expense incurred was the purchase of the high-fidelity lung model from Fasotec (Chiba, Japan), priced at 420 USD, plus shipping and customs fees. While this suggests the overall model is economically feasible, a formal cost-effectiveness analysis will be necessary to support its broader adoption and scalability. Both of these aspects—objective outcome evaluation and economic impact—will be addressed in future prospective studies currently in development.
Conclusions
Simulation-based training is an invaluable component of modern thoracic surgery education. Our three-phase training program, combining theoretical instruction, VR simulation, and hands-on practice with a physical model has provided residents with a structured, high-fidelity learning experience. The future of surgical education should focus on hybrid models, where VR-based cognitive training is complemented by physical, hands-on practice, ensuring that young surgeons gain both the notional knowledge and technical dexterity required for safe and efficient surgical performance. Further research and innovation in simulation technology will continue to refine training methodologies, ensuring the next generation of thoracic surgeons is well-prepared for the complexities of minimally invasive lung surgery.
Acknowledgments
The authors would like to express their deepest and most sincere gratitude to the Johnson & Johnson Toscana team for their unwavering support, dedication, and kindness throughout the years. Their presence and commitment have truly made a difference, enabling our ideas to grow, develop, and transform into ever-improving projects. This work would not have been possible without their invaluable contribution.
Footnote
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-16/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-16/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. No ethical approval or informed consent is required as the videos and images were produced using surgical models, not real human subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tokuishi K, Wakahara JI, Ueda Y, et al. Comparison of postoperative pain between robotic and uniportal video-assisted thoracic surgery for anatomic lung resection in patients with stage I lung cancer. Gen Thorac Cardiovasc Surg 2025; Epub ahead of print. [Crossref] [PubMed]
- Siragusa L, Angelico R, Angrisani M, et al. How future surgery will benefit from SARS-COV-2-related measures: a SPIGC survey conveying the perspective of Italian surgeons. Updates Surg 2023;75:1711-27. [Crossref] [PubMed]
- Guo C, Liu L, Zhang J, et al. Revolutionizing thoracic surgery education: a bibliometric analysis of the past decade's literature. J Cardiothorac Surg 2024;19:428. [Crossref] [PubMed]
- Hashimoto DA, Bynum WE 4th, Lillemoe KD, et al. See More, Do More, Teach More: Surgical Resident Autonomy and the Transition to Independent Practice. Acad Med 2016;91:757-60. [Crossref] [PubMed]
- Chu D, Vaporciyan AA, Iannettoni MD, et al. Are There Gaps in Current Thoracic Surgery Residency Training Programs? Ann Thorac Surg 2016;101:2350-5. [Crossref] [PubMed]
- Nashaat A, Sidhu HS, Yatham S, et al. Simulation training for lobectomy: a review of current literature and future directions†. Eur J Cardiothorac Surg 2019;55:386-94. [Crossref] [PubMed]
- Maschuw K, Schlosser K, Kupietz E, et al. Do soft skills predict surgical performance?: a single-center randomized controlled trial evaluating predictors of skill acquisition in virtual reality laparoscopy. World J Surg 2011;35:480-6. [Crossref] [PubMed]
- Jensen K, Ringsted C, Hansen HJ, et al. Simulation-based training for thoracoscopic lobectomy: a randomized controlled trial: virtual-reality versus black-box simulation. Surg Endosc 2014;28:1821-9. [Crossref] [PubMed]
- Holmstrom AL, Meyerson SL. Obtaining Meaningful Assessment in Thoracic Surgery Education. Thorac Surg Clin 2019;29:239-47. [Crossref] [PubMed]
- Reich HJ, Lou X, Brescia AA, et al. Mentorship Effectiveness in Cardiothoracic Surgical Training. Ann Thorac Surg 2021;112:645-51. [Crossref] [PubMed]
- Catasta A, Martini C, Mersanne A, et al. Systematic Review on the Use of 3D-Printed Models for Planning, Training and Simulation in Vascular Surgery. Diagnostics (Basel) 2024;14:1658. [Crossref] [PubMed]
- Hussein N, Van den Eynde J, Callahan C, et al. The use of objective assessments in the evaluation of technical skills in cardiothoracic surgery: a systematic review. Interact Cardiovasc Thorac Surg 2022;35:ivac194. [Crossref] [PubMed]
- Ghosh RM, Jolley MA, Mascio CE, et al. Clinical 3D modeling to guide pediatric cardiothoracic surgery and intervention using 3D printed anatomic models, computer aided design and virtual reality. 3D Print Med 2022;8:11.
- Cattapan C, Guariento A, Bertelli F, et al. The introduction of surgical simulation on three-dimensional-printed models in the cardiac surgery curriculum: an experimental project. J Cardiovasc Med (Hagerstown) 2024;25:165-72. [Crossref] [PubMed]
- Simi F, Fortunato GM, Diana F, et al. An Algorithm for Coding an Additive Manufacturing File from the Pressure Distribution of a Baropodometric Board for 3D Printing Customised Orthopaedic Insoles. Computation 2024;12:184. [Crossref]
- Tong BC, Gustafson MR, Balderson SS, et al. Validation of a thoracoscopic lobectomy simulator. Eur J Cardiothorac Surg 2012;42:364-9; discussion 369. [Crossref] [PubMed]
Cite this article as: Rabazzi G, Castaldi A, Aprile V, Mastromarino MG, Korasidis S, Condino S, Carbone M, Simi F, Ambrogi MC, Cigna E, Lucchi M. Training in minimally invasive thoracic surgery on 3D-model: back to the future of education. J Vis Surg 2025;11:15.



