
The far side of the moon: trans-thoracic minimally invasive resection of local recurrence of hepatocarcinoma following liver transplantation using intraoperative transdiaphragmatic ultrasound: a case report
Highlight box
Key findings
• Hepatocellular carcinoma oligorecurrence can be effectively treated with local ablative treatments and surgery.
• Intraoperative ultrasonography is extremely useful to localize subdiaphragmatic nodules through thoracoscopy allowing minimally invasive surgery.
What is known and what is new?
• Intraoperative transdiaphragmatic ultrasonography proved effectiveness in localizing hepatic nodules in several case reports.
• This is the first report of an extrahepatic nodule resection employing intraoperative ultrasonography through minimally invasive thoracoscopic surgery.
What is the implication, and what should change now?
• We here report how extrahepatic nodules deemed at high risk of complications after resection through laparotomy may be considered for excision via a trans-thoracic route. Multidisciplinary evaluation is pivotal for identifying cases suitable of highly effective and minimally invasive ablative treatments.
• Failure of a single therapeutic step should be considered as a possible scenario and should not translate into overall resignation, as long as other treatment options are viable.
Introduction
Background
Hepatocellular carcinoma (HCC) represents the most common primary liver malignancy, accounting for 75–85% of cases, and the third most common cause of cancer-related deaths worldwide (1). In patients with early-stage HCC and end-stage liver disease, liver transplantation (LT) has been proven as the best therapeutic strategy, with an excellent disease-free and overall survival rate. Nonetheless, HCC recurrence after LT is reported in 15–20% of cases (2-4), and in this case the prognosis is unfortunately poor, as it configures an advanced metastatic disease (5). The pathogenesis underlying post-transplant HCC recurrence is multifactorial but might be related to the presence of undetectable micro-metastases before transplantation or engraftment of circulating tumor cells (2).
Rationale and knowledge gap
Currently, there are no established consensus guidelines for the management of HCC recurrence after LT, mainly due to the heterogeneity and complexity of this clinical scenario (3,4) and therefore in most cases the treatment strategy must be individualized. In patients with isolated recurrence, normal allograft function, and good performance status, surgical resection or ablation with curative intent, if feasible, are an effective approach (3,4). Nevertheless, several aspects should be considered when posing a surgical indication in this context: indeed, the presence of hylar adhesions following LT may account for increased operative trauma, and the immunosuppressive therapy may increase the risk of infectious complications (5). In this perspective, choosing an effective surgical approach with the lowest invasiveness is of paramount importance, to minimize the detrimental effect of the intervention on the overall patients’ health.
Objective
We here report our experience with a patient affected by a perihepatic recurrence of HCC following LT successfully treated with several local ablative treatments, including a transthoracic surgical approach with the employment of intraoperative transdiaphragmatic ultrasound (IOTU) at S. Maria della Misericordia University Hospital. We present this case in accordance with the SCARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-4/rc).
Case presentation
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Approval was waived by our local Ethics Committee due to the nature of the report. Written informed consent was obtained from the patient for the publication of this case report, accompanying images and the video. A copy of the written consent is available for review by the editorial office of this journal.
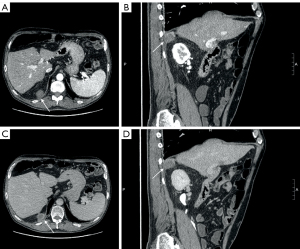
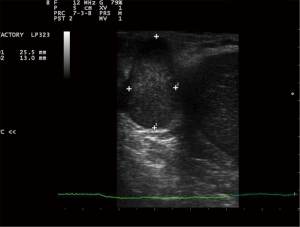
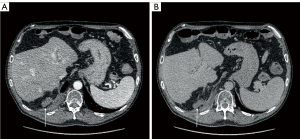
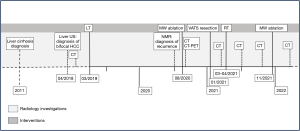
A 50-year-old male patient with a significant history of alcohol abuse was diagnosed with liver cirrhosis in 2011, with ascitic effusion as first manifestation of the disease. Routine examination unveiled portal hypertension with splenomegaly and congestive gastropathy and was classified as Child-Pugh class A (score 5). His past medical history included a smoking-related chronic obstructive pulmonary disease (COPD) and a multinodular goiter with normal thyroid function. At time of diagnosis of cirrhosis, he was not under any active drug treatment and did not undergo any previous surgical treatment. After several relapses of alcohol abuses, he later de-addicted from alcohol and tobacco consumption and was set under a regular clinical follow-up. In 2018, liver ultrasound (US) revealed the presence of two nodules which were later studied in deep with a computed tomography (CT) scan and nuclear magnetic resonance (NMR) and were confirmed to be two nodules of HCC of less than 3 cm of diameter. The patient was classified as Milan-In HCC in decompensated liver cirrhosis and therefore underwent LT in March 2019, at the age of 58 years. Histological evaluation of the explanted liver revealed signs of microvascular invasion. After LT, an immunosuppressive therapy with oral tacrolimus 6 mg once-daily (OD) and prednisone 25 mg OD was started, and the patient was set under an initial weekly and then regular monthly follow-up. The dosage of tacrolimus was tailored in the following months according to therapeutic drug monitoring (TDM) with a nadir dosage of 3 mg OD and prednisone was progressively reduced until suspension within 6 months. After an initial regular follow-up, an abdominal NMR performed in June 2020 revealed the presence of a 28-mm subdiaphragmatic nodule behind the right liver lobe, adjacent to the right diaphragmatic pillar, which was confirmed as an extrahepatic recurrence of HCC at US-guided biopsy. Therefore, immunosuppressive therapy was modified, adding oral everolimus 1.5 mg twice daily (BID) to tacrolimus to reduce its plasma levels below 5 ng/mL and the patient was initially referred to radiologist for US-guided microwave (MW) ablation (100 W/3 min, August 2020, Emprint HP Ablation System, Medtronic©, Watford, UK) and was discharged home after a 3-days uneventful post-procedural course. Meanwhile, a treatment with oral lenvatinib 8 mg OD was started. Unfortunately, the follow-up abdominal CT scan performed 2 months after the procedure (October 2020) revealed a partial response to treatment, showing that the nodule turned out hypodense apart for persistence of contrast enhancement in its upper peripheral portion (Figure 1). In the perspective of residual disease, a positron-emission tomography (PET) scan with 18F-coline was performed (October 2020), confirming a significant uptake with a standardized uptake value (SUV) max of 13.2 in the absence of further localizations (Figure 2). Contemporary, the patient presented nephrotic proteinuria and both everolimus and lenvatinib were withdrawn. In view of the failure of the ablative treatment and the persistence of a single metastatic site, the multidisciplinary board advised surgical resection of the nodule. However, due to the subdiaphragmatic posterior localization of the nodule and the previous LT, a trans-abdominal approach was deemed of unfavorable risk/benefit ratio. Therefore, a transthoracic approach was proposed. Spirometry showed almost normal lung function values. In January 2021, the patient underwent video-assisted thoracic surgery (VATS) transthoracic resection of the subdiaphragmatic HCC nodule (Video 1). After general anesthesia induction, intubation with a double-lumen tube for right lung exclusion and positioning in left lateral decubitus, a 4-cm utility port was performed in the 6th intercostal space on the middle axillary line and a further 2 cm port was created under vision in the seventh intercostal space on the posterior axillary line. Pleural cavity exploration did not identify effusion or other suspicious findings. The HCC nodule was therefore precisely localized under the diaphragm and behind the liver using an intraoperative 12 MHz laparoscopic US probe (Esaote LP323, Esaote©, Geneva, Italy; Figure 3) by the first surgeon, who had large experience with transthoracic and intraoperative ultrasonography (6). Diaphragm incision and nodule excision were performed using an US energy device (Harmonic 1100 shears, Ethicon Endosurgery© LLC, Guaynabo, PR, USA). The nodule was extracted through the utility port with a disposable bag and sent for histology. The diaphragm was repaired with a long-lasting absorbable running suture and reinforced with separated non-absorbable sutures. A chest drain tube was positioned through the lower port and the utility port closed after lung re-expansion verification. Duration of surgery was 145 minutes. The patient had an uneventful postoperative course: he was transferred to surgical ward with vital signs monitoring for the first 24 hours and routine chest X-ray and blood samples showed a regular initial post-operative course. Pain control was optimal using a continuous endovenous administration of morphine 10 mg/day and non-steroidal anti-inflammatory drugs (NSAID, ketorolac 45 mg/day) for the first 48 hours. Chest drain was removed on post-operative day 3 upon evidence of good lung expansion and low drainage output, and the patient was discharged home on post-operative day 4. Histology report confirmed HCC nodule, however with focal microscopic margin involvement. Disappointingly, the CT scan performed 2 months after surgery revealed the presence of a solid nodule with significant contrast enhancement of 45 mm of maximum diameter in the field of previous resection, expression of early local recurrence of disease. The tomographic investigation did not recognize other intra- or extrahepatic suspicious localizations. After multidisciplinary discussion, the patient was referred to radiotherapy with a dose of 64.8 Gy/27 fractions between March and April 2021. The treatment was overall well tolerated, and no adverse events were observed. However, the following CT scan revealed the persistence of the nodule with slightly increased size. A further US-guided MW ablation (100 W/6 min, December 2021) was therefore performed, and the subsequent follow-up CT scans demonstrated that the nodule turned out hypodense without contrast enhancement (Figure 4), implying effective ablation of the tumor. After a follow-up of 3 years from the last local treatment, the patient is alive, in overall good conditions and with no evidence of disease. A timeline describing graphically the overall course of the patient is reported in Figure 5.

Discussion
Key findings
We here reported a case of successful treatment of isolated HCC recurrence following LT. This clinical case is peculiar for several reasons. First, the recurrence pattern is favorably uncommon: recurrence after LT is extrahepatic in 67% of cases but usually involves distant organs such as lung, bones and adrenals (7,8), likely as a result of haematogenic spreading. While this scenario usually configures a poor prognosis, in a minority of cases with a low burden of disease and few metastatic nodules, ablative treatments may be considered and yield good survival outcomes (9,10). However, only 30% of patients with extrahepatic recurrence of disease after LT display such a recurrence pattern which may benefit from this treatment modality (5). In our case, the presence of a solitary nodule without involvement of distant organs made the condition particularly amenable of local aggressive treatments. Moreover, we described how such ablative treatments may be affected by partial failures which require re-do procedures: from our point of view, as long as there is no evidence of progressive, widespread disease, and the patient’s general conditions render repeated local procedures executable, a strict aggressive protocol should be carried on. While transparietal techniques such as MW or radiofrequency (RF) ablation represent the most employed ablative techniques in borderline subjects, thanks to their documented effectiveness and safety profile (11,12), resection still remains the preferred option in subjects with adequate fitness for surgery and completely resectable disease (13). In our case, laparotomy was not deemed feasible, due to the deep position of the nodule and the expected presence of adhesions following LT. The decision to proceed with a transthoracic approach was based on an anatomical basis and supported by the available evidence in literature. Indeed, several reports describe how tumor located in the upper and posterior segments of the liver may be resected through a transdiaphragmatic approach (14). Notoriously, when a minimally invasive approach is employed, organ palpation is hampered, and intraoperative localization techniques are necessary to identify the target nodules. Thanks to its structure, the liver parenchyma is particularly prone to ultrasonographic exploration, and the thin layer of the diaphragm does not hinder the inspection of the abdominal organs. These factors make IOTU a very useful tool for real-time localization of hepatic nodules (15,16). In this circumstance, this technique was employed to localize an extrahepatic nodule, which resulted particularly easy to identify thanks to its different echogenic pattern compared with the surrounding retroperitoneal fatty tissue. Unfortunately, our procedure resulted in specimen resection margin involvement and development of early recurrence: we do not know whether choosing a thoracotomic approach rather than VATS would have resulted in a more radical resection. In the early 2000s, Pocard and colleagues reported how the transdiaphragmatic approach to liver tumors is associated with high rates of incomplete resections, even when performed through thoracotomy (17). Furthermore, standard thoracotomy is well-known to induce a higher inflammatory response compared with VATS, a factor that may negatively affect immune tumor surveillance and the overall outcome, especially in patients under chronic immunosuppressive treatments (18). Therefore, we think that the therapeutic choices, even considering the sub-optimal results of every single step, were correct and all together contributed to the successful outcome of this case.
Strengths and limitations
There are several elements to keep in consideration while interpreting our case description. To our knowledge, this is the first case where VATS and IOTU have been employed to resect an extrahepatic recurrence of HCC. Indeed, the described pattern of recurrence is very rare, and few descriptions are available in literature. Thus, we cannot exclude a reporting bias, in that previous surgical experiences might have led to less successful results and were therefore not reported in detail. Moreover, even if histology report confirmed that the target nodule was resected with only microscopically involved margins, the size of the further recurrence observed only 2 months after VATS was quite big: this equivocal finding may induce to suspect that a further nodule might already have been present at time of surgery but not identified by US, leading to the conclusion that the accuracy of transdiaphragmatic US might be far from being optimal.
Conclusions
HCC isolated recurrence may be effectively treated with local ablative treatments, including surgical resection. Minimally invasive approaches employing US-guided precise nodule localization should be preferred, when possible, for their lower traumatic effect. Repeated procedures should be performed in case of incomplete effectiveness of treatment. Larger series are necessary to support our results.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the SCARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-4/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-4/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-4/coif). F.L. reports receiving honoraria for consulting from AstraZeneca in 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Approval was waived by our local Ethics Committee due to the nature of the report. Written informed consent was obtained from the patient for the publication of this case report, accompanying images and the video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Pravisani R, De Martino M, Mocchegiani F, et al. Recipient hepatectomy technique may affect oncological outcomes of liver transplantation for hepatocellular carcinoma. Liver Transpl 2024;30:1002-12. [Crossref] [PubMed]
- Agarwal PD, Lucey MR. Management of hepatocellular carcinoma recurrence after liver transplantation. Ann Hepatol 2022;27:100654. [Crossref] [PubMed]
- Pelizzaro F, Gambato M, Gringeri E, et al. Management of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers (Basel) 2021;13:4882. [Crossref] [PubMed]
- Badwei N. Challenges related to clinical decision-making in hepatocellular carcinoma recurrence post-liver transplantation: Is there a hope? World J Transplant 2024;14:96637. [Crossref] [PubMed]
- Londero F, Castriotta L, Grossi W, et al. VATS-US1: Thoracoscopic ultrasonography for the identification of nodules during lung metastasectomy. Future Oncol 2020;16:85-9. [Crossref] [PubMed]
- Cuadrado A, Fortea JI, Rodríguez-Lope C, et al. Risk of Recurrence of Hepatocarcinoma after Liver Transplantation: Performance of Recurrence Predictive Models in a Cohort of Transplant Patients. J Clin Med 2023;12:5457. [Crossref] [PubMed]
- Bzeizi KI, Abdullah M, Vidyasagar K, et al. Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence. Cancers (Basel) 2022;14:5114. [Crossref] [PubMed]
- Jeong YH, Hwang S, Lee GD, et al. Surgical Outcome of Pulmonary Metastasectomy for Hepatocellular Carcinoma Recurrence in Liver Transplant Patients. Ann Transplant 2021;26:e930383. [Crossref] [PubMed]
- Abdel Wahab M, Shehta A, Ibrahim EM, et al. Adrenalectomy for solitary recurrent hepatocellular carcinoma five years after living donor liver transplantation: A case report. Int J Surg Case Rep 2019;54:23-7. [Crossref] [PubMed]
- Zhai H, Liang P, Yu XL, et al. Microwave ablation in treating intrahepatic recurrence of hepatocellular carcinoma after liver transplantation: An analysis of 11 cases. Int J Hyperthermia 2015;31:863-8. [Crossref] [PubMed]
- Huang J, Yan L, Wu H, et al. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res 2016;200:122-30. [Crossref] [PubMed]
- Baz C, Nudotor R, Ian B, et al. Surgical resection of late extrahepatic metastasis of hepatocellular carcinoma 11 years after initial diagnosis: case report and literature review. J Surg Case Rep 2024;2024:rjae632. [Crossref] [PubMed]
- Aikawa M, Miyazawa M, Okamoto K, et al. Thoracoscopic hepatectomy for malignant liver tumor. Surg Endosc 2014;28:314. [Crossref] [PubMed]
- Murakami M, Aoki T, Kato T. Video-assisted thoracoscopic surgery: hepatectomy for liver neoplasm. World J Surg 2011;35:1050-4. [Crossref] [PubMed]
- Qin L, Fei L. Use of Transthoracic Transdiaphragmatic Approach Assisted with Radiofrequency Ablation for Thoracoscopic Hepatectomy of Hepatic Tumor Located in Segment VIII. J Gastrointest Surg 2019;23:1547-8. [Crossref] [PubMed]
- Pocard M, Sauvanet A, Regimbeau JM, et al. Limits and benefits of exclusive transthoracic hepatectomy approach for patients with hepatocellular carcinoma. Hepatogastroenterology 2002;49:32-5. [PubMed]
- Higuchi M, Yaginuma H, Yonechi A, et al. Long-term outcomes after video-assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg 2014;9:88. [Crossref] [PubMed]
Cite this article as: Londero F, Pravisani R, Grossi W, Cherchi VA, Bitetto D, Baccarani U, Zuin A. The far side of the moon: trans-thoracic minimally invasive resection of local recurrence of hepatocarcinoma following liver transplantation using intraoperative transdiaphragmatic ultrasound: a case report. J Vis Surg 2025;11:10.


