
Robot-assisted thoracoscopic diaphragmatic plication with absorbable mesh application: surgical technique
Highlight box
Surgical highlights
• Robotic-assisted diaphragmatic plication is a safe, feasible, and effective surgical procedure that enhances precision through advanced three-dimensional visualization and controlled tissue handling.
What is conventional and what is novel/modified?
• Conventional diaphragmatic plication has typically been performed via an open surgical approach, which requires large incisions and is associated with prolonged recovery times. In recent years, video-assisted thoracic surgery (VATS) has emerged as a widely adopted minimally invasive alternative, offering reduced morbidity and shorter hospital stays.
• Building on the minimally invasive advantages of VATS, robot-assisted diaphragmatic plication represents a further evolution in surgical technique. This approach utilizes robotic platforms to perform the procedure through small incisions, providing enhanced precision, superior visualization, and improved instrument dexterity compared to VATS.
What is the implication, and what should change now?
• Robot instruments provide enhanced precision, ensuring accurate suture placement and uniform tension.
• The technology also facilitates precise mesh positioning, offering adequate support without over-tensioning, which could hinder diaphragmatic movement.
Introduction
Background
Diaphragmatic paralysis is an uncommon acquired condition characterized by significant elevation of a hemidiaphragm without defects of continuity. In adults, the most common causes are phrenic nerve injury from cervical, cardiac or thoracic surgery, cervical trauma, tumor invasion of the phrenic nerve and idiopathic eventration in which a direct cause is not identified. The diaphragmatic eventration is more common in males and is more likely to affect the left hemidiaphragm (1) due to the absence of the liver, which is reasonably fixed.
Diaphragmatic eventration is a condition characterized by the abnormal elevation of a hemidiaphragm, resulting in significant respiratory symptoms such as dyspnea, exercise intolerance, and impaired ventilation, due to a mass effect by raised abdominal organs and to the inability of the diaphragm to promote adequate expansion of the chest and subsequent poor ventilation and impaired gas exchange. True diaphragmatic eventration is a congenital condition with development defect of the muscle or of the fibrous area and should be treated in children or newborns with the application of mesh to prevent the abdominal organ displacement. The counterpart is the diaphragmatic paralysis which is an acquired condition caused by abnormalities of the neuromuscular axis due to phrenic nerve injuries or myopathies (1). In both conditions, the weakened diaphragm raised, inducing lung atelectasis and functional impairment. The main symptom of unilateral diaphragmatic eventration is dyspnea: shortness of breath, exercise intolerance, and difficulty with sleep. Caudal movement of the diaphragm is absent, the ventilation is impaired, causing an alteration of the ventilation/perfusion ratio and then reduction of the blood gases (2).
Diaphragmatic paralysis with subsequent eventration and respiratory compromise has a huge impact on the quality of life of affected patients; these symptomatic patients can benefit from hemidiaphragmatic plication to reduce dysfunctional diaphragmatic excursion during inspiration. An isolated radiologic finding without accompanying symptoms does not warrant surgical correction (3,4).
Diaphragmatic plication can be performed by open or minimally invasive approaches through the chest or abdomen and involves gathering, reefing, and pleating the redundant segments of the hemidiaphragm to achieve tightening and flattening of the structure (2). Traditional open transthoracic plication is the most standardized approach, but the alternative thoracoscopic approach has been reported in the literature with good functional results, but functional long-term results are still lacking. The minimally invasive video-assisted thoracic surgery (VATS) approach is more complex because suturing inside the chest wall is particularly challenging (5,6). In the past 20 years, robot-assisted surgery has been successfully implemented for many thoracic procedures, showing promising results in suturing (7), and then diaphragmatic plications could be a good application of the RATS approach. Moreover, some studies reported the usefulness and effectiveness of mesh application to strengthen the diaphragm and to prevent recurrence of eventration (8,9), and in this light, the easier feasibility of suture with the robot technique allows a more precise and simpler mesh application.
Objective
The objective of this study is to report our robot-assisted technique of diaphragmatic plication and absorbable mesh application using the DaVinci Xi platform. We present this article in accordance with the SUPER reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-5/rc)
Rationale
The goal of diaphragmatic plication is to improve dyspnea by reducing dysfunctional diaphragm excursion during inspiration; hence, operative intervention is exclusively indicated for symptomatic patients (1). The robot-assisted approach can bring many advantages like increased precision, miniaturization, articulation beyond normal manipulation and three-dimensional (3D) magnification; then precision and suturing are favored, controlled and improved (3). The minimally invasive approach is feasible, safe and it is characterized by shorter hospitalization and lower morbidity and mortality rates with similar results in improving pulmonary function test (PFT) and dyspnea scores compared to open plication (10,11). The use of prosthetic mesh seems associated with a reduced incidence of post-operative events and recurrence of the eventration (9), but the use of an absorbable mesh is not well established. However, the current absorbable patches are more durable than in the past and are more tolerated and potentially less associated with complications. The rationale of applying an absorbable mesh is the collagen and scar tissue formation during the process of integration between the patch and the diaphragm, increasing the thickness and robustness of the muscle.
Preoperative preparations and requirements
Preoperative assessment included full medical history and physical examination, complete blood tests, chest computed tomography (CT), arterial blood gas analysis, complete PFT s including the measure of forced expiratory volume in first second (FEV1), forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO%) and electrocardiogram. When a diaphragmatic palsy is suspected, the patient undergoes to pre-operative dynamic chest X-ray (DCR) which is a good instrument to detect significant unilateral diaphragm dysfunction. The patient in this report was a 68-year-old male affected by hypertension, diabetes mellitus with the need for insulin and oral antidiabetic drugs; he suffered from frequent supraventricular extrasystoles in treatment with flecainide, no cervical or thoracic surgeries in the past, he had an uneventful laparoscopic cholecystectomy. In this case, the PFTs showed a restrictive pattern with FVC 2.25 L (66%), FEV1 1.72 L (64%), FEV1/FVC (98%), DLCO% (74%).
Ethical statement
This retrospective study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The retrospective analysis of the data did not require approval of the Institutional Review Board. Standard informed consent was obtained from all patients due to the non-experimental purpose of this study.
Step-by-step description
Anesthesia and patient positioning
The patient was anesthetized with intravenous general anesthesia and one-lung ventilation was obtained with a double-lumen endotracheal tube under fiberoptic bronchoscopic guidance. Invasive hemodynamic monitoring was obtained through the insertion of an arterial line. Then the patient was placed in the appropriate full lateral decubitus position, right side in this case, with the chest elevated using a sand bag (Figure 1).
Thoracic access and trocar placement
The procedure was performed using the Da Vinci Robotic Xi Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). The cart was positioned anteriorly, approximately at the pelvis, to achieve complete access to the posterior and lower part of the involved chest and hemidiaphragm.
With the patient in the appropriate decubitus, we performed an ultrasonography (Figure 2) to obtain a dynamic position of the lung and diaphragm and, based on these findings, we planned the trocar position (Figure 3). The ultrasonography is useful to verify the relationships between the diaphragm and the lung and in particular to set the first port, avoiding an injury to the diaphragm or raised abdominal organs such as liver on the right or stomach or spleen in the left. Moreover, with the use of ultrasonography, the first trocar was positioned as cranially as possible, ensuring a wide and adequate operative field.
Usually, the first port was placed in the auscultatory triangle at the fifth or fourth intercostal space and the 30-degree camera port was introduced into the pleural cavity. A significant elevation of the hemidiaphragm, particularly anteriorly, was noted, with no adhesions observed. Carbon dioxide (CO2) insufflation was used at a pressure of 6–10 cm water, and under direct visualization, two additional robot ports were created, one anterior and one posterior. An assistant port was necessary and placed between the camera and the posterior port. These capnothorax pressures have two roles: the first is to create an adequate operative field and the latter is that the diaphragmatic suture with these pressures allows a hypercorrection of the eventration, providing a greater lowering of the muscle in the post-operative period.
The robot was docked and a large needle driver and mega needle driver were inserted inon the left and right arms, respectively (Figure 4).
Diaphragmatic plication
The plication of the right hemidiaphragm was performed in a posterior-to-anterior direction using U-shaped suture with non-absorbable 1 or 2 Ti-Cron stitches on polytetrafluoroethylene (PTFE) pledgets, ensuring adequate tension in the non-pliable diaphragm. The pledgets were positioned on the superior and inferior parts of the suture, ensuring adequate stability and tissue-reinforcing on the thin diaphragm. Usually, 8–12 suture were necessary to ensure an adequate plication and tension (Video 1).
Absorbable mesh application
To strengthen the suture line and to promote scar tissue formation, an absorbable polyglactin mesh (usually 30 cm × 30 cm, doubled) was placed, anchored to the diaphragm with several interrupted 1 polyglactin sutures and secured to the chest wall with three through-and-through silk sutures.
In this phase three additional millimetric incisions were made to introduce a forceps to take out the stitches and then to close the knot in the subcutaneous layer around the rib.
If the hemi-diaphragm seems particularly floppy or frail, the BioA mesh (Gore) should be considered, but its placement and suturing is more difficult due to the more stiffness of the material and the need of more intraoperative space to correctly apply the mesh.
Closure
To check the adequate tension and plication, the CO2 insufflation was stopped and, if the plication was satisfactory, the lung was inflated to check its expansion and if the two conditions were satisfied, the robot was undocked. A chest tube was inserted above the diaphragm. The lung was fully expanded at the conclusion of the procedure. The access ports were closed, and the skin was sutured. A chest drain was connected to a three-chamber drainage system, set to suction at −20 cm water. The patient was extubated in the operating room (Figure 5).
Postoperative considerations and tasks
Following surgery, the patient was transferred to the sub-intensive care unit and then to the general ward. Generally, we scheduled some chest X-ray that showed an improvement in the elevation of the hemidiaphragm, with no signs of atelectasis and a full expansion of the lung.
The pain was managed in a standard way, including acetaminophen, tramadol, or other pain killers. The chest tube was removed when the leak output was less than 200 mL per day and sometimes, we introduced corticosteroids to reduce the liquid leak (Figures 6-9).
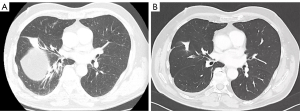
Figure 10 shows the pre-operative (CT screenshots) and 6 months post-operative follow-up CT-scan demonstrating an improved lung expansion, the absence of middle lobe atelectasis and a modified shape of the right hemidiaphragm and a remodeling of the right and left hemidiaphragm (Figure 10).
Tips and pearls
The patients’ advantages of RATS procedures are well known and RATS diaphragmatic plication has all the real benefits of RATS over other minimally invasive techniques in term of intraoperative vision, precision and handling. The last issue is basilar because suturing with the RATS technique is intuitive, easier and effective. Suture the diaphragm, adding pledgets at the edges of the suture is basilar to ensure a tight and safe plication of a tiny muscle and the redundant tissue is also useful for developing a strength barrier, reducing the risk of a recurrent eventration. In this light, the absorbable mesh promotes the inflammatory, the deposit of collagen and scar tissue formation, hardening and reinforcing the suture on the diaphragm. In this particular case, we fixed the mesh on the diaphragm and in three different points around the lowest rib, ensuring a stable and permanent position of the mesh during the degradation. Other pearls of RATS diaphragmatic plication are: the platform position set just anterior to the pelvis, the extensive use of capnothorax useful to hyper-correct the eventration, the use of ultrasonography to avoid injury to the raised abdominal viscera and the use of particular wristed instruments. In particular, we found the Mega Needle Driver and the Large SutureCut instruments very useful. The first is mandatory to arm large needle (27 mm) in a safe and stable manner and the second is very useful to reduce the assistant instrument changes (forceps, needle driver and scissors), saving time and capnothorax pressure. We equip the left robotic arm with Large SutureCut that is a wristed large needle driver with scissors in the proximal part of the instrument, and with this, we cut the stitch robotically, and then the assistant should only remove the needle.
Discussion
Surgical highlights
Robot diaphragmatic plication has become a significant advancement in the surgical treatment of diaphragmatic eventration and dysfunction. Traditionally performed using open or video-assisted thoracoscopic methods, this procedure has greatly benefited from the integration of robotic technology, particularly in terms of precision, flexibility, and enhanced visualization (12). The robot system offers superior precision and dexterity, facilitating highly accurate suturing that ensures symmetrical and properly tensioned placement of stitches, which is vital for optimal diaphragm function and long-term success, particularly in anatomically challenging regions such as the costophrenic and cardiophrenic angles (13). These areas, where the diaphragm is particularly vulnerable to eventration, benefit from the system’s ability to perform intricate maneuvers with enhanced stability and control, reducing the risk of complications during surgical intervention. To prevent the risk of eventration relapse, the application of a prosthesis patch seems advantageous even if rigorous long-term studies have never been performed (9,14,15). Non-absorbable patches have the advantage of remaining unchanged over time, but the risk of infectious complication or foreign body reactions are increased. Therefore, we prefer to insert a mesh in absorbable materials (Polyglactin, Vicryl Ethicon or BioA, Gore) that are well tolerated and integrated on the suture, strengthening the hemi-diaphragm. In our series of 7 patients who underwent diaphragmatic plication and mesh application we did not observe any sign of prosthesis infection nor relapse of the eventration. In the early post-operative period, we observe an increase in chest tube output in these patients with the need for non-steroidal or steroidal anti-inflammatory drugs.
The high-definition, 3D visualization offered by robot platforms allows the surgeon to view the diaphragm and surrounding structures with greater clarity, minimizing the risk of damage to critical structures such as the phrenic nerve, lungs, and abdominal organs—risks that are more common with open or thoracoscopic techniques.
While the robotic approach initially increases operative times, this disadvantage diminishes with experience. As familiarity with the system grows, the procedure becomes more efficient. Numerous studies highlight the safety, effectiveness, and positive immediate and short-term outcomes of robotic diaphragmatic plication (2).
Strengths and limitations
Minimally invasive RATS diaphragmatic plication can be effectively performed via a transthoracic approach, offering several advantages. This method provides a clearer surgical field, in which the view is free without the possible impediments due to the bowel, the stomach or the liver. Low-pressure CO2 insufflation aids in gently lowering the diaphragm, facilitating a tension-free plication and permitting to understanding of the real need and a direct measure of the diaphragm block on the lung. After the plication and before mesh application, it is very important to check the plication, stopping the CO2 insufflation, and if the result is not satisfactory, with the diaphragm that has been raised near the inferior pulmonary vein, for example, more sutures and stitches should be applied, ensuring a tighter and more stretched diaphragm.
Robot thoracoscopic surgery requires only small chest incisions, minimizing trauma compared to traditional open surgery. This leads to reduced postoperative pain, shorter recovery times, and faster return to normal activities. Additionally, robot diaphragmatic plication is particularly beneficial for high-risk patients, such as those with previous surgeries, obesity, or other comorbidities (16). The minimally invasive nature of the procedure lowers the risk of complications associated with larger incisions and prolonged recovery, resulting in fewer complications and a shorter hospital stay.
Robot diaphragmatic plication, while effective, presents several inherent challenges. This technique necessitates one-lung ventilation, limited spatial availability due to the constraints imposed by the ribcage and limits the surgeon’s view to only one hemidiaphragm. Furthermore, there is a risk of inadvertently incorporating bowel tissue into the plication sutures, which can lead to inadvertent injury to abdominal organs (17). In addition, RATS procedures tend to result in longer operative times, especially during the initial phase of the learning curve, although proficiency tends to reduce this over time. The cost of the procedure may be higher, but it could be offset by the potential for earlier patient discharge and faster recovery. Technically, RATS approach also involves challenges such as the absence of tactile feedback, making it more difficult to assess tissue texture, even if, after a reasonable learning curve, the surgeon learn the optic feedback that is enough to perform intracorporeal suturing in a safe manner.
Comparison with other surgical techniques and research
Diaphragmatic plication is a well-established surgical procedure aimed at restoring diaphragm function in patients with diaphragmatic paralysis or dysfunction. Traditionally, this procedure was carried out through a thoracotomy, a technique introduced by Morrison in 1923 (18).
Over time, open diaphragmatic plication (19,20) has been increasingly replaced by minimally invasive techniques, which can be performed through either transthoracic or transabdominal approaches. These approaches offer significant advantages, including reduced postoperative pain, shorter recovery times, and improved patient outcomes, leading to shorter hospital stays and enhanced quality of life. As a result, they have become the preferred choice in modern surgical practice. Although there is ongoing debate regarding the optimal minimally invasive approach—whether thoracoscopic or laparoscopic—the decision often hinges on factors such as the surgeon’s expertise, the degree of abdominal organ migration into the chest, and the presence of adhesions (21). With their observational 10-year study, Taberham et al. in 2017 (22) concluded that thoracoscopic plication of diaphragm is feasible and safe, also Gazala et al. (10) showed VATS diaphragm plication as a procedure with similar results in improving PFTs and dyspnea scores compared to open plication with shorter hospitalization and lower morbidity and mortality rates. Joseph et al. presented a single-port thoracoscopic diaphragm plication for idiopathic bilateral diaphragm paralysis as an adaptation of the conventional VATS technique (23).
However, Celik et al. in their experience with the VATS technique noted the difficulty in achieving sufficient diaphragm tension (24). Versteegh et al. found that while the incisions are smaller, there is no significant difference in the incidence or intensity of post-thoracotomy pain. Additionally, they emphasized the need for caution when operating on a paralyzed diaphragm, as it may be very thin, increasing the risk of injury to abdominal organs (25).
The limited mobility and lack of instrument articulation, combined with the use of open thoracoports and atmospheric pressure without CO2 insufflation during VATS, are hindered by the restricted workspace created by the elevated diaphragm dome. Robotic technology, with its integrated features such as instrument articulation, high-definition vision, and CO2 insufflation, offers a distinct advantage over conventional minimally invasive techniques (3,26).
At our institution, we utilize a robotic-assisted transthoracic approach for performing diaphragmatic plication. The use of robot for thoracoscopic diaphragm plication was first described in 2012 by Kwak et al. (3). Subsequently, studies conducted by Uyen-Thao et al. (11) and Schumacher et al. (27) demonstrated the safety and feasibility of RATS diaphragm plication, yielding positive results in improving patients’ pulmonary function.
In a recent single-center prospective study, Nardini et al. evaluated postoperative complications, symptomatic improvement, and changes in quality-of-life following VATS and RATS in 49 diaphragm plications, without directly comparing the two techniques. The study concluded that minimally invasive diaphragm plication is a safe procedure that leads to a rapid postoperative recovery. It is effective in alleviating debilitating dyspnea and improving quality of life (28).
Lampridis et al. conducted a single-center, retrospective comparative study involving 11 patients who underwent RATS and 9 patients who underwent open transthoracic diaphragmatic plication. The findings of the study indicate that robotic-assisted diaphragmatic plication is a safe and effective procedure, associated with a significantly shorter length of hospital stay compared to the open transthoracic approach. In the RATS group, two patients experienced partial recurrence of diaphragmatic elevation on chest CT. Although they reported less severe dyspnea compared to before the plication, they required further surgery via an open approach. No suture failures were observed, but the peripheral diaphragm was found to be thin and loose. The application was reinforced with SurgiMend or Permacol mesh (29).
We used a Vicryl absorbable mesh to cover the hemidiaphragm to avoid recurrences, generally meshes are used to correct diaphragm eventrations, congenital developmental defects in the muscular portion of the diaphragm (30), but it has been chosen for this case to prevent the postoperatively re-elevation of the diaphragm, as reported by Balci et al. They compared patients who received diaphragmatic patches during their operation (n=19) with patients receiving sole plication during their operation (n=9), none of the patients who received patches had postoperative diaphragmatic events, while two patients (22%) who received sole plication had postoperative diaphragmatic events (9). Using an absorbable material reduces the risk of infectious adverse events or foreign body reaction that could have catastrophic consequences in these patients operated for a benign but clinically impactful condition. The objective of mesh placement is scar tissue formation and thickening the diaphragm in all its areas also enforcing the peripheral region of the muscle that could lose tension and favor a recurrence over the years.
Hurley et al. reported two cases of individuals who developed diaphragmatic paralysis post-COVID-19, unresponsive to conservative management. Both patients underwent robot-assisted thoracoscopic diaphragm plication, reinforced with a bovine acellular dermal matrix. In both cases, there was significant improvement in symptoms, particularly dyspnea and fatigue (31).
Implications and actions recommended
After surgery, a routine pulmonary rehabilitation is recommended; a professional rehabilitation specialist takes charge of the patients during the hospitalization days. Aerobic exercise, walking on the ground and respiratory exercises were started on the next day after surgery to gradually achieve training objectives. After the discharge, respiratory rehabilitation, also with exercises on lateral decubitus, is recommended to better restore the activity of the patients. A 3-month follow-up with a CT scan is recommended. Further studies are needed to assess long-term efficacy and patient outcomes.
Conclusions
Robot diaphragmatic plication with mesh is an effective and minimally invasive approach to correcting diaphragmatic paralysis or dysfunction. The use of robot technology provides precision in dissection, suturing, plication, and mesh placement, while the addition of mesh could offer structural support to prevent recurrence. Surgeons can optimize surgical outcomes, reduce complications, and improve long-term respiratory function in patients undergoing this procedure.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-5/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-5/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-5/coif). The authors have no conflicts of interest to disclose.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The retrospective analysis of the data did not require approval of the Institutional Review Board. Standard informed consent was obtained from all patients due to the non-experimental purpose of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Groth SS, Andrade RS. Diaphragm Plication for Eventration or Paralysis: A Review of the Literature. Ann Thorac Surg 2010;89:S2146-50. [Crossref] [PubMed]
- Marmor HN, Xiao D, Godfrey CM, et al. Short-term outcomes of robotic-assisted transthoracic diaphragmatic plication. J Thorac Dis 2023;15:1605-13. [Crossref] [PubMed]
- Kwak T, Lazzaro R, Pournik H, et al. Robotic thoracoscopic plication for symptomatic diaphragm paralysis. J Robot Surg 2012;6:345-8. [Crossref] [PubMed]
- Kocher G, Al-Hurani M, Dorn P, et al. Thoracoscopic diaphragm plication. Multimed Man Cardiothorac Surg 2020;2020: [Crossref] [PubMed]
- Kara HV, Roach MJ, Balderson SS, et al. Thoracoscopic diaphragm plication. Ann Cardiothorac Surg 2015;4:573-5. [PubMed]
- ElSaegh MM, Ismail N, Dunning J. VATS Diaphragm Plication. Surg Technol Int 2016;28:222-5. [PubMed]
- Bongiolatti S, Annecchiarico M, Di Marino M, et al. Robot-sewn Ivor-Lewis anastomosis: preliminary experience and technical details. Int J Med Robot 2016;12:421-6. [Crossref] [PubMed]
- Kosse NJ, Galetin T, Schwarz SB, et al. Results of the Diaphragmatic Plication Database: 10 Years' Experience. Thorac Cardiovasc Surg 2023;71:483-9. [Crossref] [PubMed]
- Balci AE, Ozyurtkan MO. Clinical and surgical specifications of adult unilateral diaphragmatic eventration according to their aetiology in 28 patients. Importance of using diaphragmatic patch and minimal thoracotomy incision. Eur J Cardiothorac Surg 2010;37:606-12. [Crossref] [PubMed]
- Gazala S, Hunt I, Bédard EL. Diaphragmatic plication offers functional improvement in dyspnoea and better pulmonary function with low morbidity. Interact Cardiovasc Thorac Surg 2012;15:505-8. [Crossref] [PubMed]
- Le UT, Titze L, Hundeshagen P, et al. Robotic diaphragm plication: functional and surgical outcomes of a single-center experience. Surg Endosc 2023;37:4795-802. [Crossref] [PubMed]
- Şengül İnan M, Kavaklı K, Işık H, et al. Transthoracic robotic plication for diaphragmatic elevation. Turk Gogus Kalp Damar Cerrahisi Derg 2023;31:215-21. [Crossref] [PubMed]
- Tung KH, Yendamuri S, Seastedt KP. Adoption of the Robotic Platform across Thoracic Surgeries. J Clin Med 2024;13:5764. [Crossref] [PubMed]
- Lampridis S. Raising the bar, lowering the diaphragm: a new era in diaphragmatic plication. J Thorac Dis 2023;15:3529-32. [Crossref] [PubMed]
- Gergen AK, Stuart CM, Wojcik BM, et al. Robotic-Assisted Transthoracic Diaphragm Plication. Oper Tech Thorac Cardiovasc Surg 2023;29:216-27. [Crossref]
- Stuart CM, Wojcik BM, Gergen AK, et al. A comparison of short-term outcomes following robotic-assisted vs. open transthoracic diaphragm plication. J Robot Surg 2023;17:1787-96. [Crossref] [PubMed]
- Du VX, Groth SS. Robot-Assisted Laparoscopic Diaphragm Plication. Oper Tech Thorac Cardiovasc Surg 2022;27:449-60. [Crossref]
- Morrison JM. Eventration of diaphragm due to unilateral phrenicnerve paralysis. Arch Radiol Electrother 1923;28:72-5.
- Wright CD, Williams JG, Ogilvie CM, et al. Results of diaphragmatic plication for unilateral diaphragmatic paralysis. J Thorac Cardiovasc Surg 1985;90:195-8. [Crossref] [PubMed]
- Graham DR, Kaplan D, Evans CC, et al. Diaphragmatic plication for unilateral diaphragmatic paralysis: a 10-year experience. Ann Thorac Surg 1990;49:248-51; discussion 252. [Crossref] [PubMed]
- Di Buono G, Bonventre G, Amato G, et al. Successful laparoscopic management of congenital diaphragmatic relaxation: A case report. Int J Surg Case Rep 2020;77S:S25-8. [Crossref] [PubMed]
- Taberham RJ, Raza A, Alzetani A, et al. VATS Plication of the Diaphragm: A Descriptive Observational 10-Year Southampton Experience. Innovations (Phila) 2017;12:398-405. [Crossref] [PubMed]
- Joseph KR, Wong TS, Singh J, et al. Single port thoracoscopic diaphragm plication: A novel treatment approach to bilateral phrenic nerve palsy and diaphragm paralysis. Int J Surg Case Rep 2023;108:108387. [Crossref] [PubMed]
- Celik S, Celik M, Aydemir B, et al. Long-term results of diaphragmatic plication in adults with unilateral diaphragm paralysis. J Cardiothorac Surg 2010;5:111. [Crossref] [PubMed]
- Versteegh MI, Braun J, Voigt PG, et al. Diaphragm plication in adult patients with diaphragm paralysis leads to long-term improvement of pulmonary function and level of dyspnea. Eur J Cardiothorac Surg 2007;32:449-56. [Crossref] [PubMed]
- Gritsiuta AI, Gordon M, Bakhos CT, et al. Minimally Invasive Diaphragm Plication for Acquired Unilateral Diaphragm Paralysis: A Systematic Review. Innovations (Phila) 2022;17:180-90. [Crossref] [PubMed]
- Schumacher L, Zhao D. Outcomes and technique of robotic diaphragm plication. J Thorac Dis 2021;13:6113-5. [Crossref] [PubMed]
- Nardini M, Jayakumar S, Migliore M, et al. Minimally Invasive Plication of the Diaphragm: A Single-Center Prospective Study. Innovations (Phila) 2021;16:343-9. [Crossref] [PubMed]
- Lampridis S, Pradeep IHDS, Billè A. Robotic-assisted diaphragmatic plication: Improving safety and effectiveness in the treatment of diaphragmatic paralysis. Int J Med Robot 2022;18:e2368. [Crossref] [PubMed]
- Deslauriers J. Eventration of the diaphragm. Chest Surg Clin N Am 1998;8:315-30. [Crossref] [PubMed]
- Hurley P, Djouani A, Lampridis S, et al. Diaphragmatic paralysis post COVID-19 treated with robot-assisted plication reinforced with acellular dermal matrix: a report of two cases. Monaldi Arch Chest Dis 2022;93: [Crossref] [PubMed]
Cite this article as: Ravasin A, Burlone A, Tombelli S, Mugnaini G, Zenobi I, Salimbene O, Gatteschi L, Gonfiotti A, Voltolini L, Bongiolatti S. Robot-assisted thoracoscopic diaphragmatic plication with absorbable mesh application: surgical technique. J Vis Surg 2025;11:13.











