
Biportal robotic-assisted surgery for lung segmentectomies: a surgical technique overview
Highlight box
Surgical highlights
• Biportal robotic-assisted thoracic surgery (Bi-RATS) maintains the benefits of robotic precision and visualization while using only two incisions, improving instrument ergonomics and surgical efficiency. Compared to uniportal RATS (U-RATS), Bi-RATS eliminates the risk of crowding, improving ergonomic spacing and maneuverability of robotic arms, with low postoperative pain.
What is conventional and what is novel/modified?
• Open thoracotomy provides full access but is highly invasive. Video-assisted thoracoscopic surgery (VATS), particularly uniportal VATS, reduces trauma but has rigid instruments with limited dexterity. RATS for early-stage lung cancer is conventionally performed via four to five incisions. U-RATS minimizes access trauma but presents risk of robotic arm collisions and a time-consuming learning-curve.
• We introduced Bi-RATS as an alternative.
What is the implication, and what should change now?
• Bi-RATS provides a balanced alternative to multiport and U-RATS, enhancing procedural feasibility.
• Future research should focus on long-term outcomes, cost-effectiveness, and further refinements to solidify Bi-RATS as a less invasive approach in robotic thoracic surgery.
Introduction
Over the past two decades, minimally invasive thoracic surgery has evolved considerably, driven by the desire to reduce surgical morbidity without sacrificing oncological principles. Robotic-assisted thoracic surgery (RATS) is at the forefront of this advancement, offering high-definition, three-dimensional imaging and sophisticated, wristed instrumentation that improves ergonomics and precision (1). Traditionally, thoracic robotic procedures have relied on three- or four-port configurations. Uniportal video-assisted thoracoscopic surgery (VATS) is a feasible and safe approach with several advantages over open surgery (2), but as other thoracoscopic approaches, it comes with limitations such as rigid instruments with counterintuitive movements, fulcrum effect, and tremor amplification. These limitations can be overcome with robotic technology (3).
The growing interest in less invasive techniques has prompted the development of biportal RATS (Bi-RATS) and uniportal RATS (U-RATS) (4), which use two or a single incision, potentially reducing postoperative pain and improving patient satisfaction (5). However, U-RATS is not yet broadly recommended by manufacturers, in the same way as multiportal RATS (6), primarily due to the risk of collision between robotic arms when operating through a single access, where arms work in parallel rather than using the standard triangular configuration (triangularization), which requires a minimum distance of 3 cm to prevent interference. To address this limitation, in 2023, we established a fully Bi-RATS lung program at our centre, leveraging our extensive experience in robotic surgery and uniportal VATS (7,8).
In addition to these technical evolutions, there has been a parallel rise in the use of imaging adjuncts such as three-dimensional computed tomography (3D CT) reconstruction for preoperative planning (especially in complex segmentectomies) and near-infrared (NIR) fluorescence imaging with indocyanine green (ICG). While not mandatory for every procedure, these ancillary tools can significantly enhance lesion localization, define intersegmental planes, and confirm vascular perfusion when necessary (9). However, higher cost is the main limitation nowadays, but more studies are required to verify sustainability of robotic lobectomies. It is still possible to cover the expense with the reimbursement from Health Public System in Italy (10).
Background
Minimally invasive thoracic surgery, including robotic-assisted approaches, has revolutionized thoracic oncology and benign lung disease treatment. Compared to traditional open thoracotomy, robotic techniques offer superior visualization, enhanced dexterity, and better ergonomics. Bi-RATS combines the advantages of robotic technology with a minimally invasive approach that reduces trauma and recovery time.
Rationale
The development of Bi-RATS is driven by the need to optimize minimally invasive approaches while mitigating the limitations of uniportal VATS and multiport RATS. The ability to perform complex lung resections with robotic precision through two ports rather than three or four represents a significant advancement in the field.
Objective
This manuscript outlines the essential components of Bi-RATS for pulmonary segmentectomies, focusing on patient positioning, port placement, intraoperative technical steps, and the role of optional imaging techniques in special cases. We present this article in accordance with the SUPER reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-10/rc).
Preoperative preparations and requirements
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patient for the publication of this study, accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Adequate pulmonary reserve, acceptable cardiopulmonary status, and the absence of extensive pleural adhesions or locally advanced disease are important prerequisites. Pulmonary function testing should demonstrate a predicted postoperative (ppo) forced expiratory volume in one second (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO) ≥40% of the predicted value, in line with the established guidelines for anatomical lung resections. Patients with marginal function (ppo FEV₁ or DLCO between 30–40%) may still be candidates for segmentectomy if additional assessments, such as low-tech exercise tests (e.g., stair-climbing ≥22 m or shuttle walk >400 m) or formal cardiopulmonary exercise testing, are reassuring. Cardiac risk stratification includes a resting electrocardiogram (ECG), transthoracic echocardiography if structural heart disease is suspected, and stress testing for patients with multiple risk factors or poor functional capacity.
Preoperative imaging—especially high-resolution CT scans—allows for an accurate assessment of the lesion’s size, stage, location, and relationship to critical hilar structures. In case of complex segmental anatomy, 3D CT reconstruction can be utilized to precisely delineate the target segment(s). Additionally, patients who may benefit from intraoperative intersegmental plane demarcation can be planned for NIR fluorescence imaging with ICG, using the Firefly™ technology integrated in DaVinci Xi® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA); however, its use remains optional and dependent on institutional protocols and surgeon preference.
Step-by-step description
Operating room setup and patient positioning
A well-coordinated operating room environment is paramount for the success of Bi-RATS. The surgical team, including the anaesthesiologist, surgeon, assistants, and scrub nurse, should be thoroughly familiar with the nuances of biportal robotic approach, including robotic docking, instrumentation, and workflow coordination. Clear communication and precise role delegation among the team members are essential to ensure a smooth and efficient procedure.
After the induction of general anaesthesia (11), a double-lumen endotracheal tube is placed to enable single-lung ventilation on the operative side, providing optimal exposure of the surgical field.
The patient is then carefully positioned in the lateral decubitus, with the operative hemithorax elevated and aligned with the rest of the body in a horizontal plane (Figure 1). The ipsilateral arm is either flexed and supported or extended in a manner that avoids pressure on the brachial plexus. Careful attention to padding and stabilization—especially around the head, shoulders, hips, and knees—is critical to prevent positional injuries over the prolonged course of the operation.
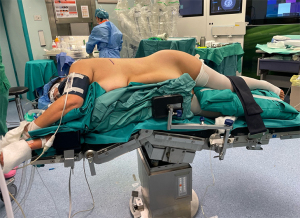
The robotic cart is stationed posterior to the patient, allowing unobstructed instrument trajectories. The assistant surgeon and scrub nurse stand at the table and play vital roles in facilitating the procedure as they are responsible for introducing or exchanging instruments through the working port, controlling suction, applying traction to enhance exposure of critical structures, and managing the retrieval of surgical specimens.
Biportal access and robotic docking
Bi-RATS is distinguished by the use of only two strategically placed incisions. One incision serves as the main working port, accommodating both the 3D robotic camera and a primary robotic instrument, typically a bipolar device. The second incision facilitates the introduction of an additional robotic arm, commonly used for deploying robotic stapling devices.
The exact intercostal spaces of these incisions may vary and be tailored based on the targeted pulmonary resection (e.g., upper vs. lower lobe) and patient-specific body habitus, but the following general principles apply (Figure 2).
- Utility incision: commonly placed in the 5th intercostal space along the midaxillary line (as in Uniportal-VATS approach). This incision is approximately 3–4 cm. The incision is fitted with a wound protector (Alexis wound retractor XS®, AppliedMedical, Rancho Santa Margarita, CA, USA). This facilitates the insertion of a robotic 30° camera in the upper part of the incision. This allows for initial exploration of the thoracic cavity and identification of any pleural adhesions, anatomical variations, or unexpected pathology prior to introducing the operative instruments. The lower portion of the utility incision accommodates one of the robotic arms. This arm is generally equipped with a Maryland bipolar dissector for procedures performed on the right side, or a fenestrated bipolar grasper when working on left side. Typically, the Maryland bipolar dissector is always assigned to the surgeon’s dominant hand at the console, so it varies based on the side of the surgery. The space between the two ports within the utility incision is used by the assistant surgeon to perform tasks as applying traction or suctioning using long and curved uniportal VATS instruments. Additionally, this incision serves as access for specimen retrieval.
- Accessory port: usually created in the 7th or 8th intercostal space along the anterior axillary line, where the intercostal space is wider. It typically accommodates a 12-mm trocar, which allows introduction of the second robotic instrument (often another bipolar device, through a trocar reducer) or robotic staplers (e.g., 30-SureForm stapler with a curved tip, a 45-stapler with a curved tip, or a 60-stapler, da Vinci® Stapling components, IntuitiveSurgical Inc., Sunnyvale, CA, USA). The lower positioning of this port facilitates proper articulation of the stapling device, enabling a fully robotic procedure, providing ergonomic benefits that reduce reliance on an assistant using manual staplers outside the robotic console and using the most advanced technology of robotic staplers.
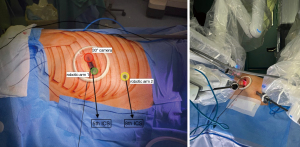
Once both utility incision and accessory port are established and their positioning verified to ensure optimal ergonomics and full range of motion of the instruments, the robotic cart is advanced and “docked” manually as in Figure 3, also shown in Video 1.

When performing surgery on right side, arm 1 is excluded, while on the left side, arm 4 is not utilised. The camera is positioned on arm 2 for a left-sided approach and on arm 3 for a right-sided approach, aligned with the lung fissure. The instruments in the other two arms are adjusted accordingly to ensure adequate spacing between the “camera” arm and the working instrument inserted through the same utility incision, as demonstrated in Video 1. Adjusting the camera vision angle from 30° up to 30°down, when working in extreme thoracic zones, can further enhance the available space between the arms.
This meticulous preparation and attention to alignment and cable organization ensure that the surgeon can operate with efficiency and without any collision of the arms during surgery.
Bi-RATS segmentectomy: intraoperative technique
The Bi-RATS workflow for thoracic surgery follows a systematic approach, regardless of whether a wedge resection, segmentectomy, or lobectomy is planned. Each procedure begins with a comprehensive exploration of the thoracic cavity and confirmation of the lesion in the planned pulmonary resection. Any adhesions are carefully dissected using the precision of robotic instruments.
Bi-RATS segmentectomy starts with the dissection of the pulmonary hilum, where the enhanced visualization and dexterity of the robotic platform facilitate isolation of lobar vessels and surrounding structures, ensuring precise separation and minimising tissue trauma. During the vascular control phase, the segmental vein and artery branches are divided using robotic staplers, requiring careful identification of accessory branches to avoid complications (Figure 4). Once vascular control is achieved, the bronchus is identified and cut.
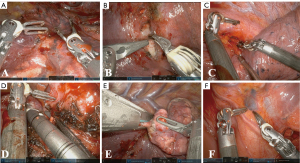
To identify the intersegmental plane, after the closure of the artery for the targeted segment, the anaesthesiologist injects 6–8 mL of diluted ICG (2.5 mg/10 mL) followed by a bolus of 10 mL saline solution. Firefly™ mode is activated and after 30–40 seconds a green glow appears from mediastinal and lung tissue, reaching the maximum intensity within a minute and fading slowly afterwards. The unperfused segment appears in grayscale, with a clear limit from the rest of the lobe, which is green coloured (Figure 5). Using bipolar forceps equipped on one arm, the operator can easily mark the intersegmental plane by spot coagulation. Afterwards, the normal light source is activated, and the intersegmental plane is stapled by 45-SureForm stapler, following the marks on the parenchyma (Video 2).

As the primary stages of the procedure are completed, attention shifts to lymphadenectomy, which involves the systematic removal of both hilar (stations 10, 11 and 12) and mediastinal lymph nodes, ensuring oncological completeness and accurate staging. These nodes are carefully dissected using robotic instruments, taking advantage of the enhanced dexterity and visualization provided by the robotic system (Video 3).
At the end of the procedure, a single 28 Fr pleural drain is placed through the lowest port incision to ensure adequate postoperative drainage as shown in Figure 6.
The typical operative time for biportal robotic-assisted segmentectomy depends on anatomical complexity and the presence of pleural adhesions. Estimated blood loss is generally low, with a median volume of 50–100 mL. These values are consistent with those reported in the literature for minimally invasive segmentectomy techniques and reflect the precision and haemostatic capabilities of robotic instrumentation.
Postoperative considerations and tasks
Following surgery, the patients are transferred to a monitored recovery setting, where pain control, respiratory function, and hemodynamic stability are closely observed.
Enhanced recovery after surgery (ERAS) protocols are implemented to expedite recovery. These include early ambulation, physiotherapy, and pulmonary exercises, as well as regional anaesthesia (e.g., nerve and fascial plane blocks), to effectively manage pain in case of necessity.
Chest tubes are generally removed once fluid drainage is minimal and radiologic imaging confirms adequate lung re-expansion. This timely removal enhances the patient’s mobility and facilitates a quicker recovery. Discharge from the hospital occurs by the third postoperative day, ensuring a smooth transition to outpatient care.
Tips and pearls
- Team coordination: ensure clear communication and role delegation among the surgical team for a smooth workflow.
- Place utility and accessory ports accurately for optimal ergonomics and instrument manoeuvrability, ensure proper spacing between robotic arms to avoid collision.
- Positioning of robotic staplers in the wider intercostal space (on the anterior axillary line) minimizes postoperative pain.
- Rapid undocking capability allows for swift conversion to uniportal-VATS or to thoracotomy in case of bleeding.
- Postoperative care: follow ERAS protocols, use regional anaesthesia, and remove chest tubes once drainage is minimal for faster recovery.
Discussion
Surgical highlights
Bi-RATS represents a significant evolution in minimally invasive pulmonary segmentectomies, combining robotic precision and visualization with the reduced access trauma of a two-port configuration. Although the learning curve can be significant—particularly for those transitioning from multiport VATS or open procedures—familiarity with robotic systems and careful case selection can facilitate a smoother adoption of this technique.
By following a structured approach to patient positioning, port placement, and intraoperative techniques, and by selectively employing imaging adjuncts such as 3D reconstructions or ICG fluorescence, thoracic surgeons can optimize outcomes across a range of pulmonary pathologies (12). Compared to fully multiport RATS—where stapling is usually performed through posterior ports—biportal RATS utilises anterior stapler access, thereby reducing mechanical stress on intercostal nerves, leading to lower postoperative discomfort. Additionally, enhanced lymphadenectomy in segmentectomy is another major advantage, as the wristed robotic instruments facilitate precise dissection of mediastinal and hilar lymph nodes, even in challenging anatomical regions.
Furthermore, advanced imaging techniques such as 3D CT reconstruction and intraoperative NIR fluorescence imaging with ICG provide real-time guidance, allowing for accurate localization of target structures and precise identification of the intersegmental plane during segmentectomy. These innovations contribute to improved oncological outcomes and safer lung-sparing procedures. As robotic thoracic surgery continues to evolve, the integration of such imaging technologies is expected to become increasingly standard, further pushing the boundaries of what is possible in minimally invasive thoracic surgery.
Strengths and limitations
Bi-RATS offers several advantages over traditional multiport approaches.
One of the primary strengths of Bi-RATS is its ability to reduce postoperative pain and improve patient recovery. Compared to multiport RATS, the lower number of ports and their anterior location have reduced intercostal nerves involvement and compression (13), leading to less postoperative discomfort and potentially a shorter hospital stay.
Another key advantage of Bi-RATS is its improved ergonomics and stapler manoeuvrability.
Bi-RATS also facilitates a more effective lymphadenectomy. The wristed robotic instruments and high-definition 3D visualization allow for precise dissection even in challenging anatomical locations. Compared to VATS, where lymphadenectomy can be limited by the rigidity of instruments and suboptimal angles, Bi-RATS enables more meticulous nodal clearance, potentially improving oncological outcomes.
Despite its advantages, Bi-RATS still faces challenges. One of the primary challenges is the learning curve associated with the technique, although less time-consuming than U-RATS. While formal comparative studies are limited, preliminary institutional experience and early reports (5,6) suggest that Bi-RATS may offer a shorter and more intuitive learning curve than U-RATS due to its more ergonomic instrument spacing and reduced risk of robotic arm collisions. For example, Yoon et al. (5) reported acceptable docking and console times after the first 10–15 Bi-RATS cases, whereas U-RATS has been shown to require at least 20–30 cases to overcome arm crowding and achieve fluency (6). Transitioning from multiport RATS or VATS requires adjustments in port positioning, robotic arm coordination, and intraoperative workflow. Surgeons must become proficient in robotic docking, instrument exchange, and procedural flow, which can initially prolong operative time. However, with experience and standardization of the technique, this learning curve can be overcome, leading to improved efficiency over time.
Another limitation of Bi-RATS is its higher cost compared to VATS. While robotic surgery provides superior ergonomics and precision, the cost of robotic platforms, instruments, and maintenance remains a significant consideration. The financial burden of robotic surgery may limit its widespread adoption, particularly in healthcare systems where cost-effectiveness studies are still ongoing. Although some countries, such as Italy, offer public healthcare reimbursement for robotic lobectomies, the long-term sustainability of Bi-RATS will depend on further economic evaluations and comparisons with other minimally invasive approaches.
Despite these limitations, Bi-RATS remains a good compromise and evolving technique that bridges the gap between multiport RATS and uniportal VATS, without the potential limits discussed for U-RATS. As robotic technology continues to advance, further comparative studies, cost analyses, and surgical refinements will help define the long-term role of Bi-RATS in modern thoracic surgery.
Comparison with other surgical techniques and research
Several studies have suggested that port placement plays a crucial role in postoperative pain following RATS. The findings by Tokuishi et al. (13) highlight that posterior port positioning, particularly in patients with a smaller thoracic cavity, can lead to increased postoperative discomfort due to greater mechanical strain on the chest wall and intercostal nerves. The dorsal intercostal space is generally narrower, making it more susceptible to instrument-induced stress and nerve compression, particularly when robotic arms operate at a more orthogonal angle to the ribs. This mechanical load is further evidenced by the higher incidence of bleeding observed at dorsal port sites, suggesting increased trauma to intercostal structures during surgery. According to our preliminary experience, Bi-RATS, with its more anterior port configuration, may offer an advantage in reducing postoperative pain, particularly in patients with smaller thoracic dimensions. Furthermore, the anterior placement of ports allows for a more ergonomic trajectory of robotic instruments, minimizing excessive stress on the intercostal spaces while still preserving the benefits of enhanced dexterity and visualization. While further comparative studies are necessary, these findings support the rationale for adopting a more anteriorly positioned, minimally invasive approach to optimize patient comfort and recovery outcomes.
Moreover, this observation is supported by recent findings from Ning et al. (14), who demonstrated that postoperative pain in robotic thoracic surgery is influenced by port positioning, with greater pain reported when instrumentation and drainage tubes are placed in the posterior intercostal spaces. Their study suggests that posterior ports may increase intercostal nerve compression and mechanical load, particularly in patients with smaller thoracic cavities. Conversely, a more anterior port configuration may alleviate these issues by allowing better distribution of instrument movement and reducing direct pressure on the intercostal nerves.
Compared with multiportal RATS, one of the notable advantages of Bi-RATS is its rapid undocking capability, which allows for swift conversion to uniportal VATS or open thoracotomy in the event of complications such as major bleeding. This flexibility is particularly important in situations where quick intervention is required, as it minimizes downtime and allows the surgical team to address emergent issues effectively without compromising patient safety. Bi-RATS offers a balanced approach, combining the minimal invasiveness of a two-port technique with the flexibility to undock and convert quickly, thereby reducing the risk of delays during critical situations. This is particularly valuable in complex or high-risk procedures, where the operating team must be prepared for unforeseen complications.
Additionally, the ergonomic benefits of Bi-RATS allow for a fully robotic stapling process, reducing reliance on manual staplers that could generate additional trauma through excessive manipulation. One of the recognized challenges in U-RATS is the technical difficulty in vascular stapling, primarily due to the limited manoeuvrability within a single access point and the dimensions of the robotic staplers. The need to accommodate larger robotic staplers in a confined space can prolong operative time, particularly when dealing with upper lobe artery branches or smaller-calibre vessels, where precise angulation and stapler positioning are essential. The literature highlights that in U-RATS, achieving an optimal stapler trajectory requires extensive countertraction on the lung parenchyma, increasing surgical complexity and potentially affecting efficiency (15). Furthermore, U-RATS requires modified, thinner trocars to reduce external conflicts between robotic arms, which may not always be readily available, increasing procedural complexity. Without these adaptations, the risk of instrument crowding and arm collision becomes a significant limiting factor, particularly for vascular stapling (15).
In contrast, Bi-RATS eliminates these concerns by providing dedicated access points for different instruments, allowing better spacing and reducing the risk of robotic arm conflict. In our experience, vessel isolation is straightforward and consistently achieved without vessel tension or damage due to a more direct and controlled trajectory of the stapler introduced in the second anterior port towards the hilar structures.
For smaller vessels, such as left upper lobe artery branches or minor right upper lobe branches, the use of robotic Hem-o-lock clips can be a valuable alternative, as they are smaller and easier to introduce into the chest. While the 45 SureForm robotic stapler is technically feasible in U-RATS, its use is often restricted due to difficulties in approaching vessels and the increased risk of external conflicts between robotic arms. The 30 EndoWrist curved tip stapler could be beneficial in overcoming these limitations, but its unavailability has been a noted restriction (15).
Implications and recommended actions
As robotic thoracic surgery continues to evolve, Bi-RATS is poised to become an important mainstay of modern thoracic surgical practice. The combination of anterior stapler access, improved ergonomics, and the latest robotic stapler technology results in faster and more efficient vascular division, reducing intraoperative challenges.
Future studies should focus on further refining this technique, improving cost-effectiveness, and conducting comparative analyses with multiport RATS and U-RATS to establish clear clinical guidelines. Additionally, as robotic technology advances, the integration of augmented reality overlays, artificial intelligence-assisted imaging, and real-time perfusion assessments could further enhance surgical precision and patient outcomes.
By adopting Bi-RATS as a standard minimally invasive approach, thoracic surgeons can optimize surgical planning, reduce postoperative pain, and improve the efficiency of complex pulmonary resections, ultimately leading to better patient care and surgical outcomes.
Disclaimer
The surgical techniques detailed in this manuscript should be adapted to each patient’s specific anatomy and clinical scenario, as well as the operating surgeon’s experience and institutional resources. Surgeons who are new to Bi-RATS may benefit from mentoring or proctorship to ensure safety and efficacy during the learning curve.
Conclusions
Bi-RATS is an innovative approach that combines the benefits of robotic precision with a less invasive two-port configuration, offering improved ergonomics, reduced access trauma, and enhanced surgical efficiency in lung segmentectomy. Learning curve and cost remain key considerations, and further studies are needed to validate long-term oncological and functional outcomes.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Takuya Watanabe) for the series “Uniportal Video-Assisted Thoracoscopic Segmentectomy for Early-Stage Non-Small Cell Lung Cancer” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-10/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-10/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-25-10/coif). The series “Uniportal Video-Assisted Thoracoscopic Segmentectomy for Early-Stage Non-Small Cell Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patient for the publication of this study, accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu X, Wang M. Efficacy and Safety of Robot-assisted Thoracic Surgery (RATS) Compare with Video-assisted Thoracoscopic Surgery (VATS) for Lung Lobectomy in Patients with Non-small Cell Lung Cancer. Comb Chem High Throughput Screen 2019;22:169-78. [Crossref] [PubMed]
- Nachira D, Congedo MT, Tabacco D, et al. Surgical Effectiveness of Uniportal-VATS Lobectomy Compared to Open Surgery in Early-Stage Lung Cancer. Front Surg 2022;9:840070. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Ning Y, Chen Z, Zhang W, et al. Short-term outcomes of uniportal robotic-assisted thoracic surgery anatomic pulmonary resections: experience of Shanghai Pulmonary Hospital. Ann Cardiothorac Surg 2023;12:117-25. [Crossref] [PubMed]
- Yoon J, Park SI, Lee GY, et al. Dual-portal robotic-assisted thoracic surgery for early-stage non-small cell lung cancer: A feasibility study. J Thorac Dis 2021;13:6351-9.
- Stamenovic D, Schiller P, Karampinis I, et al. Uniportal robotic assisted surgery for anatomical lung resection-First German experience. Int J Med Robot 2023; Epub ahead of print. [Crossref] [PubMed]
- Kuzmych K, Sassorossi C, Nachira D, et al. Fully Dual-Portal Robotic-Assisted Thoracic Surgery (F-DRATS) and Indocyanine Green-Navigated Segmentectomy. Surg Tech Dev 2024;13:294-300. [Crossref]
- Nachira D, Meacci E, Congedo MT, et al. eComment. Uniportal VATS: the great potential of the technique. Interact Cardiovasc Thorac Surg 2017;25:163. [Crossref] [PubMed]
- Hsieh CP, Liu YH, Wu YC, et al. Indocyanine green fluorescence-navigated robotic segmentectomy. Surg Endosc 2017;31:3347-8. [Crossref] [PubMed]
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Punzo G, Beccia G, Cambise C, et al. Goal-Directed Fluid Therapy Using Pulse Pressure Variation in Thoracic Surgery Requiring One-Lung Ventilation: A Randomized Controlled Trial. J Clin Med 2024;13:5589. [Crossref] [PubMed]
- Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [PubMed]
- Tokuishi K, Wakahara JI, Ueda Y, et al. Factors related to post-thoracotomy pain following robotic-assisted thoracic surgery. Asian J Endosc Surg 2024;17:e13302. [Crossref] [PubMed]
- Ning Y, Chen Z, Zhang W, et al. Short-term outcomes of uniportal robotic-assisted thoracic surgery anatomic pulmonary resections: experience of Shanghai Pulmonary Hospital. Ann Cardiothorac Surg 2023;12:117-25. [Crossref] [PubMed]
- Mercadante E, Martucci N, De Luca G, et al. Early experience with uniportal robotic thoracic surgery lobectomy. Front Surg 2022;9:1005860. [Crossref] [PubMed]
Cite this article as: Kuzmych K, Nachira D, Congedo MT, Calabrese G, Senatore A, Margaritora S, Meacci E. Biportal robotic-assisted surgery for lung segmentectomies: a surgical technique overview. J Vis Surg 2025;11:11.



