Depicting surgical anatomy of the porta hepatis in living donor liver transplantation
Background
In the United States, approximately 17,000 patients are on liver transplant waiting lists, but only about 6,000 transplants are performed per year (1). The majority of transplant liver grafts are of deceased donor origins, with less than 0.03% living donor liver transplants (LDLT). The limiting factors of deceased donor grafts include organ shortages, compatibility issues and the time-sensitive nature of the surgery. LDLT reduces waiting time for the recipient, provides an opportunity for pre-surgical planning and compatibility matching, and increases the likelihood of a successful transplant while at the same time expanding the donor pool (2).
While liver transplantation is often the best treatment option for patients with end-stage acute or chronic hepatic disease, vascular complications following transplantation may hamper long-term success (3). These complications have been reported to have an incidence rate as high as 9% (4). Minimization of these complications is imperative considering the organ shortages and long waiting lists for liver transplantation.
The “complex anatomic architecture” of the vascular and biliary structures at the porta hepatis makes it a surgically challenging area (5). Vascular and biliary structures of the porta hepatis are highly variable and can impact clinical outcomes (6-10). When considering vascular structures, Hiatt et al identified 6 variations of hepatic artery branches supplying the liver, (n=1,000), whereas Michels et al. identified 10 (n=200) (11). Atasoy et al. concluded from a study of 200 patients that variations in main portal vein (MPV) branching are frequent (12). At least eight variations of the course and pattern of entry of the cystic duct into the common hepatic duct have been described (13), with hepatic biliary anatomy classified as “non-traditional” in as much as 42% of the population (6).
Vascular and biliary injuries of the porta hepatis during LDLT surgery may not only result in complications, but have also been reported to be fatal in as many as 40–50% of cases (14,15). In addition, Strasberg et al. have suggested, “the most common cause of serious biliary injury is misidentification” (16).
Visualizing the complex anatomy of the vascular and biliary structures of the liver on a case-by-case basis is essential to the preoperative planning and successful surgery. In this paper a LDLT right hepatectomy case, with focus on the porta hepatis, will be used to demonstrate an innovative method to visualize the relevant anatomy not entirely captured by imaging technology. This is achieved by integrating pre-surgical planning, case-specific footage and 3D anatomical reconstructions.
Methods
The donor for this right hepatectomy was an adult female. The objective was to procure the right lobe of the donor’s liver, which would be transplanted into a recipient with primary biliary cirrhosis. Permission for filming was granted through the standard Consent to Treatment form (Form 2019A), signed by the patient as required by University Health Network.
Donor preoperative workup included contrast-CTs of venous and arterial phases, and MRI. Cholangiograms were obtained intraoperatively to inform surgical decision-making. During the preoperative planning phase, surgeons evaluated the abdominal imaging series, and reconstructed the data into 3D models using Intrasense Myrian® software (17). This visualization application allows for viewing of the patient’s anatomy in fully rotatable models, where key anatomical landmarks can be identified. The 3D reconstructions were also used for volume calculations, to ensure the graft from the donor was of appropriate size for the recipient, and the remnant liver in the donor was adequate to ensure postoperative liver function.
From the imaging series and other pre-surgical testing, the surgeons developed a written script to outline the surgical plan. This script was distributed to the attending residents, operating room (OR) nursing staff, and the medical illustrators.
Selected segments of the footage, and accompanying 3D animation were posted online after several months of production, in an open-access video library created by the medical illustrators and surgeons: the Toronto Video Atlas of Surgery (TVASurg). TVASurg is a unique resource that allows surgeons and medical trainees not only to review complicated procedures, and also to apply core principals to other cases.
Prior to surgery, the medical illustrators were briefed on the case, and arrangements were made with the OR nursing staff to set up for filming. For filming, it is necessary to have a mounting bracket for an overhead camera, and a sterile Thompson™ flexible endoscope holder for an infield camera (Figure1A). The sterile Thompson™ holder was mounted to the retractor ring system on the operating table by the surgeons at the start of the procedure. The camera housing units were designed specifically for this purpose and fabricated by the Medical Engineering Department at Toronto General Hospital.
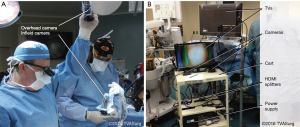
On the day of the procedure, the medical illustrators ensured that the mounting bracket for the overhead camera was in place. The filming cart had two 24” TV screens, and two Sony HXR-MC1 Point of View camera systems enclosed in sterile endoscope sleeves, along with twelve 16 GB Sony Pro-HG Duo memory cards, two HDMI splitters, six HDMI cables, and an isolated power transformer (Figure1B). Once the patient was anesthetized, the medical illustrators brought the filming cart into the OR and assisted the surgeons with the setup of the infield and overhead cameras.
The surgery was filmed from the initial skin incision to closure. The overhead camera mounted above the OR table and infield camera mounted to the retractor ring system of the OR table enable dynamic capture of the surgery from varying angles. Audio capture was used throughout the surgery, as the surgeons pointed out relevant structures and discussed the reasons for any deviations from the initial surgical plan. A time log was used to document the key surgical events during the procedure to provide a timeframe reference for editing. Photographs were taken in the OR to capture lighting conditions and provide contextual reference for the video. Also, the liver graft was photographed after removal.
Postsurgery, the memory cards were transferred to a workstation, and the time log and photographs archived. Footage was edited in Adobe Premiere Pro, and later composited using Adobe after Effects (San Jose, California, USA). CTs were imported with Intrasense Myrian® (Montpellier, France), to reconstruct the anatomy in exportable 3D meshes (OBJ format) for retopology and animation in Maxon Cinema 4D (Friedrichsdorf, Germany). After the animation shots were rendered in high resolution, all production assets including the footage, animations, overlays, labels, captions and narration audio were composited in Adobe after Effects.
Results
The anatomy and relationships of the structures of the porta hepatis including biliary ducts, hepatic arteries and portal veins, were defined preoperatively from CT imaging and further delineated during the surgery. Direct observation of the dissected porta hepatis during surgery provided the opportunity to compare preoperative images to the structures encountered in vivo.
The preoperative imaging for this case included venous and arterial contrast CTs. These were reconstructed into 3D models and used for analyzing the branching patterns of the portal veins (Figure 2) and hepatic arteries (Figure 3).
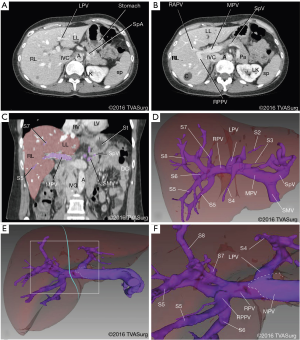
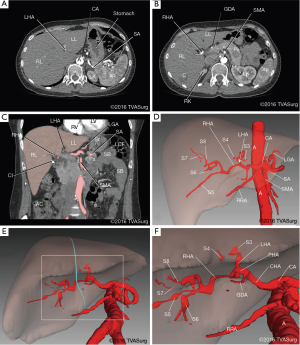
Portal veins
The MPV was formed by the merging of the splenic and superior mesenteric veins. At the porta hepatis, the MPV gave off the left portal vein (LPV), and then continued into the substance of the liver as the right portal vein (RPV). Close to its origin, the RPV gave off a small vein, the “annoying vein”, supplying segment 6 (Figure 4). This vessel, emerging from the posterior aspect of the RPV, was isolated and ligated to safely mobilize the RPV, to prevent tearing and subsequent bleeding.
The LPV divided into branches to segments 2, 3, and 4, and then coursed inferiorly in the left lateral fissure as the pars umbilicus, a remnant of the prenatal ductus venosus. The distal end of the LPV is continuous with the round ligament of the liver, containing the obliterated left umbilical vein. The LPV will be the main portal blood supply to the recipient’s remnant left lobe.
The RPV divided into anterior and posterior trunks. Two segment 5 branches were observed, one from each trunk. In this patient, the anterior trunk supplied segments 5 and 8 and the posterior trunk supplied segments 5, 6, and 7 (Figure 2D,F).
Hepatic arteries
The common hepatic artery (CHA) arose from the celiac trunk and, after giving off the gastroduodenal artery (GDA), became the hepatic artery proper (HAP). The HAP bifurcated into left hepatic arteries (LHA) and right hepatic arteries (RHA) prior to entering the porta hepatis, medial to the bifurcation of the MPV. The LHA was short, and divided into segmental branches supplying, sequentially, segments 4, 2 and 3. The RHA was longer, and gave off an independent segment 5 branch proximally. As it continued to course postero-superiorly, the segment 6 branch was given off, and subsequently the artery bifurcated into segment 7 and 8 branches (Figure 3D,F).
Bile ducts
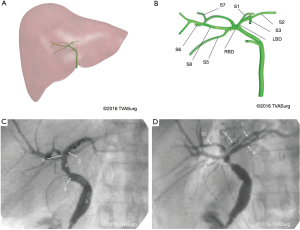
The bile ducts were not adequately visualized with contrast-enhanced CTs or preoperative cholangiograms to allow for 3D model reconstruction, and therefore their location was determined from the pattern of portal vein distribution, supplemented with the operative cholangiogram. In this case, the donor had undergone a previous cholecystectomy, indicated by a metal clip on the remaining cystic duct. Cholangiograms were performed during surgery to provide visualization of the entire biliary tree (Figure 5A,B) and inform surgical exposure.
Surgery
In the OR, the right hepatic artery was the first to be dissected in the porta hepatis. This was followed by mobilization of the RPV. Because this patient had undergone a previous cholecystectomy, no gall bladder was present, and the remnant cystic duct was used to insert a 4-French PD catheter for a cholangiogram (Figure 5C,D). During porta dissection, a metal clip was placed on the hilar plate at a proposed location for the right bile duct (RBD). A staff radiologist was called into the OR to perform an intraoperative cholangiogram, and supported the preoperative anatomy findings: the RBD was very short, existing for only 1–2 mm from the divergence of the left bile duct (LBD) and nearby caudate duct (Figure 5B).
To ensure the best possible post-surgical outcome, division of the biliary plate would be distal to the bifurcation of the segment 5/8 and segment 6/7 bile ducts, resulting in two duct orifices to be reconstructed in the recipient, but keeping the bifurcation and LBD in the donor intact.
Transection & graft retrieval
With porta dissection complete, the right lobe was mobilized off the diaphragm and the small segment 6 and 7 veins to the caval were divided. The line of parenchymal dissection was drawn on the liver surface using electrocautery, running from the groove between the right hepatic veins (RHV) and middle hepatic veins (MHV) superiorly. On the inferior surface, transection will pass through the gall bladder fossa towards the metal clip on the hilar plate. An umbilical tape is looped through the R-MHV groove and posterior to the liver to serve as a guide for transection, and assist with elevating the liver (the “hanging manoever”).
Parenchymal transection was undertaken using water jet dissection and electrocautery. Larger vascular structures were ligated with silk ties and cut with scissors, while smaller branches were ligated with metal clips and divided using cautery. Once parenchymal transection was complete, the right lobe remained attached only by vascular and biliary structures. A second cholangiogram was done to verify biliary anatomy, and confirmed that division would take place on the right side of the metal clip, resulting in two bile ducts on the right lobe liver graft.
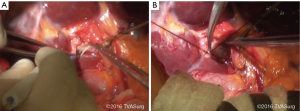
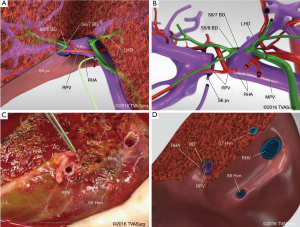
The biliary plate and RBD within were divided sharply with scissors, and each resulting open duct was probed to confirm the anatomy (Figure 6A,B). The duct orifices and biliary plate on the donor site were oversewn with a 6–0 absorbable suture. After coordination with the recipient transplant team, the anaesthetist administered heparin to the patient and the remaining vascular structures were divided: the RHA was ligated with silk suture and divided; the RPV and RHV were ligated using an endovascular stapler and divided sharply using scissors. The right lobe graft was immediately removed, placed in an ice bucket and flushed with cold preservation solution (Figure 6C,D).
Video production
The donor right hepatectomy procedure was filmed from skin incision to closure, for a total of 5 hours and 49 minutes of uninterrupted footage from two recording sources. From this raw footage, the medical illustrators produced an initial 8-minute edit for review by the surgeon. Next, the 3D models were constructed and prepared for animation. The 3D content was added to the revised footage, both in the form of animated demonstration segments, and still “overlay” illustrations integrated with the footage. All stages of video production served useful for teaching and contributed to preoperative planning of similar cases. The completed video was then posted online (http://TVASurg.ca).
Conclusions
The video produced for the Toronto Video Atlas of Liver Pancreas and Transplant Surgery (TVASurg.ca) is a unique resource that is based on surgical footage and operative imaging, enhanced with patient-specific 3D animation. This patient-specific approach provides a clinically valuable teaching tool since all production assets are based on clinical events, without anatomical or narrative idealizations.
A main focus of this video was the donor porta hepatis anatomy as it guides surgical decision-making and impacts outcomes. LDLT procedures differ from liver resections in that the graft must be kept viable, and therefore the porta structures are left intact until removal. When the porta structures are divided during a LDLT hepatectomy, each will be ligated leaving an open-ended blood vessel or bile duct that can be reconstructed in the recipient.
The smaller branches of the portal venous system may pose bleeding risk or other complications if not identified prior to ligation and division. The segment 6 portal vein, the “annoying vein” as described by surgeons, may be difficult to locate, since it branches from the posterior branch or posterior aspect of the RPV. It is important to divide this vessel without damaging the RPV. Similarly, another small vein, while not encountered in this case, supplying the caudate lobe, may be found branching from the posterior surface of the LPV. The segment 6 vessel was seen in the operative footage and then demonstrated at higher magnification in the animation-enhanced video to emphasize their importance.
During the editing process, the 5+ hours of surgical footage was edited to a <10-minute sequence to highlight the most important steps of the surgery. To prevent disorientation, colour-coded overlays and targeted magnification were utilized. The use of overlay illustrations clarifies not only what is seen in the operative field, but can also demonstrate to the viewer, the origin and course of segmental branches and reveal their detailed spatial relationships through the use of techniques such as transparency. The 3D overlays, integrated with the surgical footage, permit visualization of the structures of the porta hepatis and intrahepatic anatomy from any view. Traditional illustration techniques are static and therefore limited in the number of views, and the ability to show deformation of structures. 3D models, in addition to providing unlimited viewing angles and the ability to animate deformations, also enable visualization of any anomalies, based on patient anatomy.
Anatomy of the porta hepatis is frequently taught through illustrations, as photographs of dissections from both cadavers and the OR can be difficult to interpret. Bile ducts and hepatic arteries in particular can have indistinguishable diameters and course in similar directions. As is the case with many anatomical illustrations intended for basic education, simplification of structures may result in subtle errors that affect the interpretation of the viewer. Contemporary uses of 3D models in digital teaching tools, such as apps for smart phones or tablet devices, often include over-generalizations and low-resolution 3D models. Integrating customized and high fidelity 3D models with footage retains clinically relevant features and directs the viewer’s focus within a real-world narrative framework.
The script prepared by the surgeons, sequentially outlining the steps of the procedure, presents an opportunity for clarification and discussion of the case by the surgical team. From the filming perspective, the medical illustrators reviewed the script with the surgeons to optimize camera angles and inform the filming process. The preoperative planning and video production both use the same 3D reconstruction software, making a seamless transition from the planning to production phases (18-21). Since no two cases are identical, each case requires careful planning to ensure that all steps of the procedure are captured as re-filming is not possible.
The TVASurg has been a valuable resource since its inception 5 years ago as evidenced by the continuously growing global user base. Presently, site traffic is at about 330 visitors per day. The website is intended for surgeons, fellows, residents, medical students, OR nursing staff and others. In the atlas, each animation-enhanced video case also includes the relevant medical history, imaging and links to related videos. In two cases, both the donor and recipient transplant procedures are documented. Clinicians who have visited the site have stated that the atlas “combine(s) technical innovation with a deep knowledge of surgery,” “is helpful in “understand(ing) how to deal with complex (surgical) situations,” and “is saving time and money.”
The 3D reconstructed models have also been adapted for a patient education section of the site. Patients and family members have found this section to be “excellent and very educational” in providing a comprehensive overview of the procedure. Both clinicians and patients have found the educational approach of the animation-enhanced videos to be informative and visually engaging.
In conclusion, the novel educational system described in this paper enables integrating case-based operative footage with advanced editing techniques for visualizing surgical procedures, as well as the complex anatomy of the liver vascular and biliary structures. The clinically based design approach results in a final product that is archived in an open-access online surgical video atlas. The utilization of a narrative approach combined with cinematic production techniques allows for seamless sequential organization from pre-surgical planning to outcome.
Acknowledgements
The authors would like to thank the Biomedical Communications program at the University of Toronto-Mississauga, and operating room staff at Toronto General Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- United Network for Organ Sharing, UNOS regional meeting Data. Richmond, VA; 2014. Available online: https://www.unos.org/data/
- Sureka B, Bansal K, Rajesh S, et al. Imaging panorama in postoperative complications after liver transplantation. Gastroenterol Rep (Oxf) 2016;4:96-106. [Crossref] [PubMed]
- Caiado AH, Blasbalg R, Marcelino AS, et al. Complications of liver transplantation: multimodality imaging approach. Radiographics 2007;27:1401-17. [Crossref] [PubMed]
- Duffy JP, Hong JC, Farmer DG, et al. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg 2009;208:896-903; discussion 903-5. [Crossref] [PubMed]
- Tirumani SH, Shanbhogue AK, Vikram R, et al. Imaging of the porta hepatis: spectrum of disease. Radiographics 2014;34:73-92. [Crossref] [PubMed]
- Catalano OA, Singh AH, Uppot RN, et al. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics 2008;28:359-78. [Crossref] [PubMed]
- Covey AM, Brody LA, Getrajdman GI, et al. Incidence, patterns, and clinical relevance of variant portal vein anatomy. AJR Am J Roentgenol 2004;183:1055-64. [Crossref] [PubMed]
- Erbay N, Raptopoulos V, Pomfret EA, et al. Living donor liver transplantation in adults: vascular variants important in surgical planning for donors and recipients. AJR Am J Roentgenol 2003;181:109-14. [Crossref] [PubMed]
- Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 1994;220:50-2. [Crossref] [PubMed]
- Strasberg SM, Eagon CJ, Drebin JA. The "hidden cystic duct" syndrome and the infundibular technique of laparoscopic cholecystectomy--the danger of the false infundibulum. J Am Coll Surg 2000;191:661-7. [Crossref] [PubMed]
- Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966;112:337-47. [Crossref] [PubMed]
- Atasoy C, Ozyürek E. Prevalence and types of main and right portal vein branching variations on MDCT. AJR Am J Roentgenol 2006;187:676-81. [Crossref] [PubMed]
- Lamah M, Karanjia ND, Dickson GH. Anatomical variations of the extrahepatic biliary tree: review of the world literature. Clin Anat 2001;14:167-72. [Crossref] [PubMed]
- Jurkovich GJ, Hoyt DB, Moore FA, et al. Portal triad injuries. J Trauma 1995;39:426-34. [Crossref] [PubMed]
- Pearl J, Chao A, Kennedy S, et al. Traumatic injuries to the portal vein: case study. J Trauma 2004;56:779-82. [Crossref] [PubMed]
- Strasberg SM, Brunt LM. Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg 2010;211:132-8. [Crossref] [PubMed]
- Fung A, Kelly P, Tait G, et al. Creating an animation-enhanced video library of hepato-pancreato-biliary and transplantation surgical procedures. J Vis Commun Med 2016;39:27-32. [Crossref] [PubMed]
- Ballantyne L. Comparing 2D and 3D imaging. J Vis Commun Med 2011;34:138-41. [Crossref] [PubMed]
- Jurgaitis J, Paskonis M, Pivoriūnas J, et al. The comparison of 2-dimensional with 3-dimensional hepatic visualization in the clinical hepatic anatomy education. Medicina (Kaunas) 2008;44:428-38. [PubMed]
- Kong X, Nie L, Zhang H, et al. Do Three-dimensional Visualization and Three-dimensional Printing Improve Hepatic Segment Anatomy Teaching? A Randomized Controlled Study. J Surg Educ 2016;73:264-9. [Crossref] [PubMed]
- Su L, Dong Q, Zhang H, et al. Clinical application of a three-dimensional imaging technique in infants and young children with complex liver tumors. Pediatr Surg Int 2016;32:387-95. [Crossref] [PubMed]
Cite this article as: Kelly P, Fung A, Qu J, Greig P, Tait G, Jenkinson J, McGilvray I, Agur A. Depicting surgical anatomy of the porta hepatis in living donor liver transplantation. J Vis Surg 2017;3:43.


