Surgical and oncological outcomes of thoracoscopic thymectomy for thymoma
Introduction
Thymoma remains the most common primary anterior mediastinal neoplasm (1,2). Surgical resection remains central to the treatment, and multimodality therapy, including radiation and/or chemotherapy, is indicated as adjuvant therapy (3-5). Total thymectomy via standard median sternotomy is the standard treatment for early and advanced stage thymoma (6,7). With recent developments in thoracoscopic surgical instruments and practices, thoracoscopic procedure has been increasingly indicated for thymoma (7-9). Recent studies have shown that thoracoscopic thymectomy (TT) improves surgical outcomes, including reduced postoperative pain, fewer postoperative complications, and shorter postoperative hospital stay, due to the decreased invasiveness of the procedure (10,11). Furthermore, equivalent oncological outcomes have been demonstrated for TT compared with open thymectomy (OT) (7,10,11). However, currently, several controversies exist over the applicable surgical approach, surgical criteria, operative indication of thoracoscopic procedure, and the resected extent of the thymus. Additionally, there are no crinical guidelines of TT for thymoma. This present review article aimed to summarize current studies comparing TT and OT.
Thymoma
Thymoma is usually an indolent disease. Epithelial tumors of thymus generally involve thymoma, thymic carcinoma, and carcinoid. Thymomas are associated with autoimmune diseases, such as myasthenia gravis (MG), hypogammaglobinemia, and pure red cell aplasia. Thymic carcinoma and carcinoid are very rare neoplasms (2). Most patients with thymomas are asymptomatic; however, a few patients experience systemic symptoms including a cough, dyspnea, and autoimmune disease. Thymomas may cause local symptoms associated with compression of surrounding structures. Approximately 30% of patients with thymomas have MG (12), and around 20% of patients with MG have thymomas (13). Thymomas are generally considered cytological benign whereas thymic carcinomas have malignant cytological features. Thymic carcinomas have a significantly worse oncological prognosis (14-16).
Clinical stage
Bergh et al. (17) presented the initial clinical staging for thymoma in 1978, which was subsequently altered by Wilkins and Castleman (18). Masaoka et al. established an alternative clinical staging system (6) in 1981 and the Masaoka stage is widely accepted based on clinical treatments (Table 1). Recently, the Masaoka-Koga stage (19) was shown as a modified clinical staging system by the International Thymic Malignancy Interest Group (ITMIG). The Masaoka stage is recognized as an effective prognostic factor for thymoma (16). Several reports have shown that the oncological outcomes significantly correlated with Masaoka stages (16,20-23). In our previous study (24), Masaoka stage III–IV thymoma patients experienced more recurrences than those with stage I–II patients. The 5-year disease-free survival (DFS) for stage III–IV thymomas was significantly poorer than those for stage I and II thymoma (47% vs. 97.5% and 94.1%, respectively). Hence, the Masaoka stage was a valuable predictor of the prognosis of thymoma.
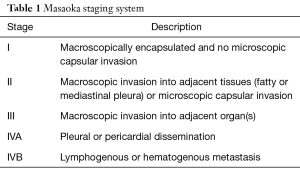
Full table
Pathologic classification
The pathologic classification of thymoma has remained controversial for many years (25). The World Health Organization (WHO) has developed the most widely adopted pathologic classification for epithelial tumor of thymus by taking into consideration both histological and morphological features (26). There are two staging systems for epithelial tumor of thymus (27). Most widely used clinical stage was first described by Masaoka et al. in 1981, and it is a clinical staging system describing thymomas in terms of local extension or invasion of the tumor (6). The TNM staging follows the pattern of T for tumor descriptor, N for nodal spread, and M for distance metastasis. In 1999, the WHO Consensus Committee published a histologic typing system for neoplasms of the thymus (28). Thymomas are classified into types A, AB, B1, B2, B3, and C based on the morphology of epithelial cells and ratio of lymphocyte-to-epithelial cells. Recently, the WHO Consensus Committee recommended that epithelial tumors of thymus be classified as thymoma, including type A, AB, B1, B2, and B3 thymoma and thymic carcinoma (29). Most of the WHO type A, AB, B1, and B2 thymomas are benign; however, types B3 and C have malignant potential (30). Our study (24) demonstrated that 16 of 140 thymomas patients experienced recurrences. Of those 16 patients, 12 patients had WHO type B3 or C thymomas. Thus, pathological aggressiveness seems to strongly influence recurrence. Several reports have indicated the impact of the WHO type on the decision-making during clinical treatments (26).
Surgical treatment
Most patients having Masaoka stage I–II thymoma receive surgical treatment. Recently, TT has been attempted as a surgical treatment for thymoma. TT for thymoma consists of extended thymectomy (ET), total thymectomy, subtotal thymectomy (STT), and partial thymectomy according to the classification of the extent of the thymus resected. In this review, a few authors classified thymectomy by total and partial thymectomy, while some authors do not describe the resected extent of thymus clearly. Total thymectomy was defined as the resection of the entire thymus, and ET was defined as the resection with the thymus and surrounding fat tissues together. ET was usually performed in patients who had MG. For these patients, thoracoscopic ET was performed using a bilateral thoracic approach in our institution. STT was defined as the resection of the thymus without the superior and inferior poles in a contralateral thymic lobe (31). Total thymectomy and STT using a unilateral thoracic approach are the main thoracoscopic procedures for thymoma in our institution (Figure 1). Resection is initiated in the pericardial fat and normal thymic tissues with careful handling of the tumor to evade disruptions into the capsule of thymoma. This thoracoscopic technique ensures a satisfactory view to the surgeon despite deep and narrow spaces. In addition, multiple thoracoscopic instruments support the technical difficulties.

Surgeons who favor the right-side thoracic approach state that the superior vena cava can be obviously recognized and used as a marker to dissect around the left innominate veins. Surgeons who use the bilateral thoracoscopic approach advocate that it improves the visualization of main anatomical structures and consequently enables complete resection. Hence, the laterality of approach depends on the surgeon or institution indications, which are influenced by their experience and training (33). Furthermore, some institutions use a sternal lifting (34) or carbon dioxide insufflation (35,36). Recently, most thymoma patients indicated TT in our institution. Most reports show that patients with Masaoka stage I–II thymomas underwent TT (37). The invasion to the innominate vein, phrenic nerve, or other major vessels should be a contraindication to a TT (36). Patients with Masaoka stage III thymoma should be indicated for OT.
In agreement with previous reports (12,38,39), several authors demonstrated the decreased invasiveness of TT for thymoma. Most of the reports in this review revealed that TT showed a significantly shorter post-operative hospital stay, decreased intraoperative blood loss, and fewer complications compared to OT (Table 2).
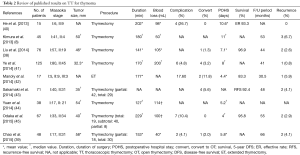
Full table
Concern regarding the chance of thymoma capsule rupture and risk of pleural dissemination by the thoracoscopic approach has been raised. The thymoma should not be grasped directly with instruments because of these risks. Exact gentle handling should be recommended (36). However, this concern is not confirmed by published evidence (46). Kimura et al. (8) reported that three (6.7%) of 45 patients with thymoma >5 cm who underwent a thoracoscopic approach had disease recurrence with pleural dissemination. Lucchi et al. reported that pleural recurrence after TT was comparable to that after OT via sternotomy (47). The recurrence ranged from 0% to 6.7% with variable follow-up periods (30–66 months). Patients who experienced recurrence tended to have Masaoka stage III–IV or WHO type B3-C thymoma. The 5-year DFS ranged from 83.3% to 96% (Table 2). According to recent reports, surgical treatment for thymoma demonstrated that the overall 5-year survival rate of patients with Masaoka stage I and II thymoma ranged from 89% to 100% and 71% to 95%, respectively (48). Ye et al. (41) demonstrated that the oncological outcomes were comparable for TT and OT groups. Manoly et al. (42) concluded that the DFS was comparable between the groups. Several reports demonstrated that there was no significant difference in the DFS between the groups (43,49).
No definitive guidelines have yet been established regarding the appropriate size of the thymoma that can be considered an indicator for thoracoscopic surgery. Minimal invasive surgery for a relatively large-sized thymoma remains controversial. There is presently no consensus on the appropriate size of the thymoma for which TT can be performed, and there are few reports that have examined the indications for TT based thymoma size. Most investigators accept that TT is technically safe and feasible for thymomas with a diameter <50 mm (37,50).
Concern regarding local recurrence or intrathymic relapse may limit the indications of limited resections including STT, partial thymectomy, and thymomectomy for Masaoka I–II stage thymoma without MG because the appropriate resected extent of the thymus is uncertain. No definitive guidelines have yet been established regarding the resected extent of the thymus for thymoma. Unnecessary, extensive resection of the thymus may increase the potential risk of the intra and post-operative complications. In our previous report, there was no significant difference in the DFS classified by the resected extent of the thymus (45). Thoracoscopic STT was the most frequently performed procedure in our department may have contributed to decreasing the potential risk with oncological equivalent. Sakamaki et al. (51) showed that partial thymectomy was feasible for the treatment of noninvasive thymoma without MG. There were no cases of marginal or mediastinal recurrence during the follow-up period. They also suggested that partial thymectomy with negative surgical margins does not carry a greater risk of recurrence than total thymectomy.
Post-thymectomy MG occurs in around 1–3% of the operated patients, and this disorder even progresses after ET (52), suggesting that extended total thymectomy is not beneficial in avoiding post-thymectomy MG over STT.
Tseng et al. (53) demonstrated that there was no significant difference in the recurrence rate between patients who received ET and those who received partial thymectomy. Singhal et al. (54) concluded that complete resection of thymoma was sufficient surgical treatment for Masaoka stage I–II thymoma. Several authors have reported that complete resection of thymoma is essential of the treatment for thymoma (51,55). Several studies demonstrated that complete resection is one of critical prognostic factor in the treatment of thymoma (4,22). Hence, complete resection is required for surgical treatment of thymoma. It will become necessary to determine an appropriate resection extent of the thymus.
Conclusions
TT is less invasive with oncological equivalent outcomes compared to OT. The Masaoka stage and WHO type classification were valuable predictors of the prognosis of thymoma. The optimal treatment for thymoma should be performed according to the Masaoka stage and WHO type classification. Minimally invasive surgery, including TT for stage I–II thymomas, is becoming the mainstay of therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kuwano H, Amano J, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2010 Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2012;60:680-708. [Crossref] [PubMed]
- Shimosato Y, Mukai K, Matsuno Y. Tumors of the mediastinum. In: Silverberg SG. editor. AFIP atlas of tumor pathology, 4th series, fascicle 11. Washington, DC: Armed Forces Institute of Pathology, 2010.
- Shields TW. Thymic tumors. In: Shields TW, editor. Mediastinal surgery. Philadelphia: Lea & Febiger, 1991:153-73.
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84. [Crossref] [PubMed]
- Hejna M, Haberl I, Raderer M. Nonsurgical management of malignant thymoma. Cancer 1999;85:1871-84. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Kondo K. Therapy for thymic epithelial tumors. Gen Thorac Cardiovasc Surg 2014;62:468-74. [Crossref] [PubMed]
- Kimura T, Inoue M, Kadota Y, et al. The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2013;44:e214-8. [Crossref] [PubMed]
- Ye B, Li W, Ge XX, et al. Surgical treatment of early-stage thymomas: robot-assisted thoracoscopic surgery versus transsternal thymectomy. Surg Endosc 2014;28:122-6. [Crossref] [PubMed]
- Zahid I, Sharif S, Routledge T, et al. Video-assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:40-6. [Crossref] [PubMed]
- Ng CS, Wan IY, Yim AP. Video-assisted thoracic surgery thymectomy: the better approach. Ann Thorac Surg 2010;89:S2135-S2141. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Mao ZF, Mo XA, Qin C, et al. Incidence of thymoma in myasthenia gravis: a systematic review. J Clin Neurol 2012;8:161-9. [Crossref] [PubMed]
- Levine GD, Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol 1978;9:495-515. [Crossref] [PubMed]
- Mayer R, Beham-Schmid C, Groell R, et al. Radiotherapy for invasive thymoma and thymic carcinoma. Clinicopathological review. Strahlenther Onkol 1999;175:271-8. [Crossref] [PubMed]
- Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg 2004;77:1183-8. [Crossref] [PubMed]
- Bergh NP, Gatzinsky P, Larsson S, et al. Tumors of the thymus and thymic region: I. Clinicopathological studies on thymomas. Ann Thorac Surg 1978;25:91-8. [Crossref] [PubMed]
- Wilkins EW Jr, Castleman B. Thymoma: a continuing survey at the Massachusetts General Hospital. Ann Thorac Surg 1979;28:252-6. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies clarification and definition of terms. J Thorac Oncol 2011;6:S1710-S1716. [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908-13; discussion 914. [Crossref]
- Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg. 2004;77:1860-9. [Crossref] [PubMed]
- Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32. [Crossref] [PubMed]
- Odaka M, Shibasaki T, Kato D, et al. Comparison of oncological results for early- and advanced-stage thymomas: thoracoscopic thymectomy versus open thymectomy. Surg Endosc 2017;31:734-742. [Crossref] [PubMed]
- Shimosato Y. Controversies surrounding the subclassification of thymoma. Cancer 1994;74:542-4. [Crossref] [PubMed]
- Detterbeck FC. Clinical value of the WHO classification system of thymoma. Ann Thorac Surg 2006;81:2328-34. [Crossref] [PubMed]
- Sellke FW, del Nido PJ, Swanson SJ. editors. Sabiston and Spencer's Surgery of the Chest. 8 ed. Philadelphia: Elsevier, 2010:2520.
- Rosai J. Histological typing of tumours of the thymus. In: International histological classification of tumours, 2nd ed. New York: Springer, 1999.
- Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and genetics of tumours of the lung, pleura, thymus and heart. In: WHO classification of tumours, 2nd ed. Lyon: IARC Press, 2004:145-97.
- Lee HS, Kim ST, Lee J, et al. A single institutional experience of thymic epithelial tumours over 11 years: clinical features and outcome and implications for future management. Br J Cancer 2007;97:22-8. [Crossref] [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [Crossref] [PubMed]
- Odaka M, Tsukamoto Y, Shibasaki T, et al. Thoracoscopic subtotal thymectomy (STT) for thymoma. In this case, STT from left thoracic cavity for a large thymoma. Asvide 2017;4:147. Available online: http://www.asvide.com/articles/1455
- Tomulescu V, Popescu I. Unilateral extended thoracoscopic thymectomy for nontumoral myasthenia gravis--a new standard. Semin Thorac Cardiovasc Surg 2012;24:115-22. [Crossref] [PubMed]
- Aubert A, Chaffanjon P, Brichon PY. Video-assisted extended thymectomy in patients with thymoma by lifting the sternum: is it safe? Ann Thorac Surg 2004;77:1878. [Crossref] [PubMed]
- Petersen RH. Video-assisted thoracoscopic thymectomy using 5-mm ports and carbon dioxide insufflation. Ann Cardiothorac Surg 2016;5:51-5. [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-S1742. [Crossref] [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [Crossref] [PubMed]
- Liu TJ, Lin MW, Hsieh MS, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol 2014;21:322-8. [Crossref] [PubMed]
- Chao YK, Liu YH, Hsieh MJ, et al. Long-term outcomes after thoracoscopic resection of stage I and II thymoma: a propensity-matched study. Ann Surg Oncol 2015;22:1371-6. [Crossref] [PubMed]
- He Z, Zhu Q, Wen W, et al. Surgical approaches for stage I and II thymoma-associated myasthenia gravis: feasibility of complete video-assisted thoracoscopic surgery (VATS) thymectomy in comparison with trans-sternal resection. J Biomed Res 2013;27:62-70. [Crossref] [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-1603. [Crossref] [PubMed]
- Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg 2014;45:e187-93. [Crossref] [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-1237.e1. [Crossref] [PubMed]
- Yuan ZY, Cheng GY, Sun KL, et al. Comparative study of video-assisted thoracic surgery versus open thymectomy for thymoma in one single center. J Thorac Dis 2014;6:726-33. [PubMed]
- Odaka M, Shibasaki T, Asano H, et al. Feasibility of thoracoscopic thymectomy for treatment of early-stage thymoma. Asian J Endosc Surg 2015;8:439-44. [Crossref] [PubMed]
- Raza A, Woo E. Video-assisted thoracoscopic surgery versus sternotomy in thymectomy for thymoma and myasthenia gravis. Ann Cardiothorac Surg 2016;5:33-7. [PubMed]
- Lucchi M, Davini F, Ricciardi R, et al. Management of pleural recurrence after curative resection of thymoma. J Thorac Cardiovasc Surg 2009;137:1185-9. [Crossref] [PubMed]
- Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. [Crossref] [PubMed]
- Tagawa T, Yamasaki N, Tsuchiya T, et al. Thoracoscopic versus transsternal resection for early stage thymoma: long-term outcomes. Surg Today 2014;44:2275-80. [Crossref] [PubMed]
- Takeo S, Tsukamoto S, Kawano D, et al. Outcome of an original video assisted thoracoscopic extended thymectomy for thymoma. Ann Thorac Surg 2011;92:2000-5. [Crossref] [PubMed]
- Sakamaki Y, Kido T, Yasukawa M. Alternative choices of total and partial thymectomy in video-assisted resection of noninvasive thymomas. Surg Endosc 2008;22:1272-7. [Crossref] [PubMed]
- Kondo K., Monden Y. Myasthenia gravis appearing after thymectomy for thymoma. Eur J Cardiothorac Surg 2005;28:22-5. [Crossref] [PubMed]
- Tseng YC, Hsieh CC, Huang HY, et al. Is thymectomy necessary in nonmyasthenic patients with early thymoma? J Thorac Oncol 2013;8:952-8. [Crossref] [PubMed]
- Singhal S, Kaiser LR. Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg 2003;76:1635-41; discussion 1641-2. [Crossref]
- Kohman LJ. Controversies in the management of malignant thymoma. Chest 1997;112:296S-300S. [Crossref] [PubMed]
Cite this article as: Odaka M, Tsukamoto Y, Shibasaki T, Mori S, Asano H, Yamashita M, Morikawa T. Surgical and oncological outcomes of thoracoscopic thymectomy for thymoma. J Vis Surg 2017;3:54.


