Left video-assisted thoracoscopic enucleation of a giant horseshoe oesophageal leiomyoma
Introduction
Oesophageal leiomyoma, with an overall incidence of less than 50/10,000 in autopsy series, is a rare condition, albeit being the most common benign tumour of the oesophagus. Approximately 50% of patients are asymptomatic with a diagnosis made on incidental discovery. When symptomatic, symptoms include dysphagia, retrosternal pain, heartburn, regurgitation or occasionally upper gastrointestinal bleeding or weight loss (1,2). They are twice as common in men and are usually found in the middle to distal third of the oesophagus. In the distal oesophagus, leiomyomas may reach a large size and cause encroachment on the cardia of the stomach (3). Most cases in the past have been managed by open resection, and in certain cases requiring a partial oesophagectomy with or without colonic interposition (4). These patients require prolonged hospitalization and a long recovery time. We report successful enucleation of a giant horseshoe oesophageal leiomyoma via a thoracoscopic approach and the management of an intra-operative complication of a mucosal breach.
Pre-operative work-up
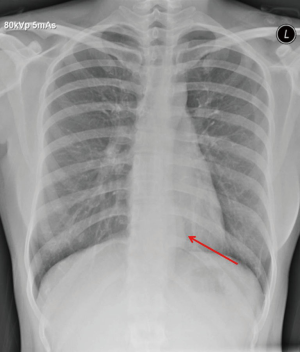
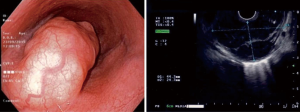
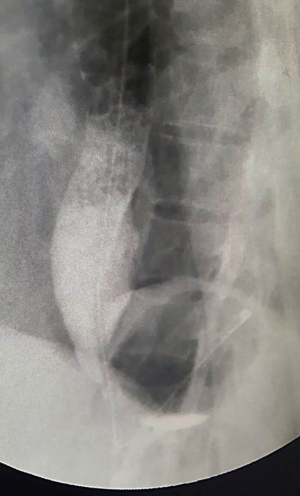
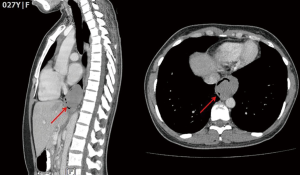
An asymptomatic 27-year-old woman was diagnosed with an oesophageal leiomyoma which was discovered incidentally on routine chest radiograph (Figure 1). Computed tomography (CT) of the thorax showed a 4.0 cm × 3.6 cm × 5.5 cm polypoid soft tissues mass in the distal third of the oesophagus, causing narrowing and deviation of the lumen anteriorly (Figure 2). Endoscopy revealed a large subepithelial mass at the distal third of the oesophagus, 1cm proximal to the gastro-oesophageal junction. The lesion was imaged as a 45 mm × 25 mm hypoechoic and inhomogeneous mass arising from the muscularis propria on endoscopic ultrasound (Figure 3). Fine needle aspiration (FNA) of the lesion was performed. Cytology came back as spindle cells with no cytological atypia or mitotic activity evident, with cells staining positive for Desmin and negative for CD117, DOG-1 and S100 on immunohistochemistry; consistent with a leiomyoma.
Equipment preference card
- 5 mm 30 degree camera lens;
- 5mm Maryland dissector;
- 5 mm endoscopic suction apparatus;
- 5 mm endoscopic bipolar energy source;
- Endoscopic “peanut” dissector;
- 5 mm endoscopic needle holder;
- 10 mm endoscopic retrieval bag.
Operative procedure
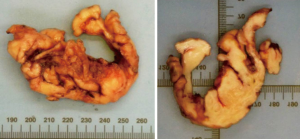
The operation was done with the patient in right lateral decubitus position. The patient was intubated with a double-lumen endotracheal tube, allowing for single lung ventilation. 3 ports were inserted along the left mid-axillary line; a 5-mm instrument port in the 5th intercostal space, a 5-mm camera port in the 6th intercostal space and a 10-mm multiple instrument port in the 7th intercostal space (Figure 4). The last port was used for chest tube placement at the end of the operation. The full operative procedure is described in the accompanying video (Figure 5). Total operation time was 4 hours with an estimated blood loss of 50 mL. Final histology of the specimen revealed a 12 cm × 3 cm × 3 cm leiomyoma with multiple fimbriae (Figure 6).

Post-operative management
The chest drain was removed on post-operative day (POD) 1. In view of the mucosal breach and repair, the patient was kept nil-by-mouth and was started on bolus nasogastric feeding on POD 1. She was discharged home on POD 3. Contrast swallow performed on POD 6 did not demonstrate a leak, with no hold-up and good flow of contrast into the stomach (Figure 7). As such, she was started on a liquid diet. This was tolerated well and diet was escalated accordingly. She was back to eating a normal diet at 3 weeks post-operation. She remains well at 6 months’ follow-up.
Discussion
We postulate that the breach in the mucosa occurred at the previous FNA site. If an oesophageal leiomyoma is suspected, many do not recommend diagnostic punctures of the lesion when the mucosa appears intact on endoscopy as pre-operative diagnostic punctures can cause obliteration of the layers of the oesophageal wall, resulting in difficulties during surgical enucleation and increased risk of mucosal perforation (4,6), as was in our case. In addition, the gain of these biopsies may be low, even when performed with endosonographic guidance, due to inadequate depth of puncture or necrotic centers in large leiomyomas.
Tips and tricks
- If a leiomyoma is suspected, biopsy is avoided to prevent mucosal scarring and subsequent adhesions between the tumour and mucosa. If needed, endoscopic ultrasound-guided FNA should be done to limit mucosal breach and scarring. It also prudent to wait a few weeks after biopsy for mucosal healing before surgery;
- For lower third leiomyomas near the gastro-oesophageal junction, the left-sided video-assisted thoracoscopic surgery (VATS) approach is superior to a laparoscopic approach as it gives better exposure and access to the distal oesophagus. Exposure can be further enhanced by extending the dissection right down to the hiatus and using crural traction sutures;
- Traction sutures aid in creating adequate tension of tissue planes for dissection, however care must be taken to avoid over-retraction to prevent “cheese-wiring” through soft lesions and delicate tissues;
- Unlike in well encapsulated leiomyomas, in horseshoe-shaped leiomyomas with multiple fimbriae, the whole oesophagus, proximally and distally as well as circumferentially around the tumour should be mobilized. This allows for easy enucleation of the leiomyoma and its many corkscrew finger-like extensions extending around the oesophagus;
- After enucleation of the tumour, it is mandatory to check carefully for mucosal breach either by direct visual inspection or by inflating air under water. If detected, it should be repaired and the patient not fed orally for 5 days;
- The muscle layer should always be approximated to prevent future pseudo-diverticulum formation.
Conclusions
Pre-operative diagnostic punctures of benign oesophageal lesions may result in difficulties during surgical enucleation and may increase the risk of mucosal perforation. Even so, when performed by experienced thoracic surgeons, video-assisted thoracoscopic enucleation of giant oesophageal leiomyomas is feasible and safe and avoids prolonged hospitalization, morbidity and poor cosmetic outcome associated with open surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Aurea P, Grazia M, Petrella F, et al. Giant leiomyoma of the esophagus. Eur J Cardiothorac Surg 2002;22:1008-10. [Crossref] [PubMed]
- Stadler J, Orda R, Baratz M, et al. Giant leiomyoma of the esophagus as a cause for gastrointestinal bleeding. J Clin Gastroenterol 1987;9:613-5. [PubMed]
- Priego P, Lobo E, Alonso N, et al. Surgical treatment of esophageal leiomyoma: an analysis of our experience. Rev Esp Enferm Dig 2006;98:350-8. [Crossref] [PubMed]
- Rijcken E, Kersting CM, Senninger N, et al. Esophageal resection for giant leiomyoma: report of two cases and a review of the literature. Langenbecks Arch Surg 2009;394:623-9. [Crossref] [PubMed]
- Chan EE, Agasthian T. Left video-assisted thoracoscopic enucleation of a giant horseshoe oesophageal leiomyoma. Asvide 2017;4:193. Available online: http://www.asvide.com/articles/1501
- Simoglou C, Mikroulis D, Konstantinou F, et al. Large diameter oesophageal leiomyomas. Hellenic J Surg 2015;87:268-70. [Crossref]
Cite this article as: Chan EE, Agasthian T. Left video-assisted thoracoscopic enucleation of a giant horseshoe oesophageal leiomyoma. J Vis Surg 2017;3:63.