Thymic minimally invasive surgery: state of the art across the world—Europe
Introduction
The landscape of different surgical approaches for the surgical treatment of thymic malignancies is rapidly changing and—as it historically did—still harbors a wide range of different possible approaches. The choice of surgical approach is influenced by the evolution of evidence based advances in thymic surgery but also historical development of the institutional technique, technological advances, socioeconomic constraints and the surgeons’ skills, previous experiences and personal preferences.
Thoracic surgeons went with the times and incorporated technical achievements, such as video-assisted thoracic surgery (VATS) and robotic-assisted thoracic surgery (RATS) into their practice of thymic surgery. Their learning abilities for new technologies contributed to smaller incisions, better cosmetic results and patient comfort, without compromising oncologic radicality and patient safety.
International initiatives of the European Society of Thoracic Surgeons [ESTS (1)] Thymic Working Group, the International Thymic Malignancies Interest Group [ITMIG (2)] and the Japanese Association for Research on the Thymus [JART (3)] have leapfrogged the development of surgical achievements in thymic surgery. Nevertheless, the introduction of minimally invasive techniques has brought new aspects into the ongoing debate on the “best” surgical approach for thymic malignancies. Surgeons experienced in traditional open thoracic surgery favor sternotomy or thoracotomy and express concerns about the possible risk of more recurrences because of inferior control of resection margins, damage to the capsule (desmoplastic reaction) that covers most thymomas and seeding of malignant cells with endoscopic manipulations. One might not forget that endoscopic manipulations are controlled by vision of 2D- or 3D-screens with the only tactile feedback reaching the surgeons’ fingers through long instruments during VATS procedures or no tactile feedback at all during RATS surgery. Surgeons performing minimally-invasive thymic surgery always have to keep in mind that abandoning the option of viewing the operative field directly as well as having tactile feedback to their fingers from exploring the patients’ tumor, nerves or great vessels with their fingertips may lead to a loss in valuable information necessary for optimal surgical judgement which may hamper patient safety, oncologic or myasthenic outcomes in advanced tumor surgery or unexpected anatomic difficulties. Many of nowadays concerns will find their answers when long term follow up data on recurrences and survival in terms of open and minimally invasive thymic surgery become available. This article focuses on the peer reviewed literature on minimally invasive thymic surgery published by European thoracic surgery centers.
What is the dimension of minimally-invasive thoracic surgery in Europe?
According to the thymoma section of the ESTS annual database report 2015, 17.6% of thymic surgery was operated by VATS, 3% by VATS-robotic and 1.9% by a transcervical approach (4,5). In a cohort study of the ESTS Thymic Working group on 229 thymic carcinomas in the ESTS retrospective database from 1990 to 2010 seven percent of patients had surgery by a VATS or RATS approach (6).
We will use the common designations for the minimally-invasive procedures that were used in previous classifications for thymic surgery even anatomic descriptors (e.g., subxyphoid) are intermingled with terms of the technical equipment used for the procedure (e.g., robotic, Table 1). Future classifications of terms describing thymic MIS will take into account that technical equipment used for VATS or RATS can be inserted through any anatomic site (e.g., subxyphoidal or transcervical).
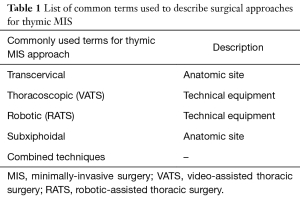
Full table
Different surgical approaches published by European centers
Transcervical approach
Reports of cervical thymectomy on patients with myasthenia gravis range back to the surgeon Ernst Ferdinand Sauerbruch [1912 (7)]. After promising results were published in the early 80’s (8): the cervical approach became again attractive for surgeons dealing thymic pathologies. Sternal lifting with retractors facilitated the surgical access to the anterior mediastinum (9).
Excellent results of radical thymic resections with low operating costs and no necessity for sternal lifting were recently reported with the use of a V-shaped sternal retractor allowing partial sternotomy from the 4–5 cm cervical skin incision (10).
Another possible valid indication for transcervical approaches was described by Zieliński et al. regarding re-thymectomy in patients who suffered previous non complete thymic resections (11).
VATS approach
After the implementation of thoracoscopic procedures in General Thoracic surgery at the end of the 90’s, some European centers, started successful programs including thymic resections. Most of the early published European series started their thoracoscopic thoracic surgery programs with the treatment of non-thymomatous myasthenia gravis (12,13). Not all technical refinements of VATS thymectomy over time can be mentioned here, but techniques were steadily improved, e.g., the preoperative induction of an adjuvant pneumomediastinum to ease visualization and mobilization of the thymus (14).
VATS thymectomy was more and more proposed as a valid less invasive alternative to open approaches. Left-sided VATS thymectomy was reported to achieve improvement or remission in 95% of patients with myasthenia gravis [1993–1997, 31 patients, 4 patients had thymomas, Vergata University, Rome, Italy, and Catholic University, Leuven, Belgium (12)].
Further debates about the proper side—left or right—to perform the operation took place in the last 20 years. Many institutions preferred a tumor orientated approach—left or right sided approach depending on the predominant laterality of the thymoma (15). Others followed a bilateral thoracoscopic approach when difficulties in visualizing the contralateral side were encountered (e.g., the contralateral phrenic nerve). In a series 50 patients undergoing right-sided VATS resections for Masaoka-Koga stages I and II, a bilateral VATS approach was necessary in 6 patients [Istanbul University, Turkey (16)]. Glancing at the peer reviewed published literature one might get the impression that there is a preference for a left-sided approach in European high volume centers of thymic surgery while in large American and Asian series the right-sided approach seems more prevalent.
New concepts incorporating the latest technological advances in minimally-invasive surgery pave the way for changing thymic surgery. Video-assisted thoracoscopic microthymectomy serves as a magnificent example [James Cook University Hospital, Middlesbrough, UK (17)].
RATS approach
The start of a “robotic era” became reality in Europe in the beginning of the 21st century (18,19). Institutions from several European countries reported promising results in the treatment of mediastinal disorders. Early experiences of RATS on the resection of mediastinal masses including thymomas were reported from the University of Innsbruck [Austria, 2001–2003, 14 patients (20)] or thymectomy in patients with myasthenia gravis from the University of Padua [Italy, 2002–2004, 33 patients (18)].
Studies comparing RATS with sternotomy favored the RATS approach in quality of life [Antwerp University Hospital, Belgium, 2004–2008, 14 RATS vs. 22 sternotomies (21)], cosmetic effects, pain, and shorter hospital stays [University of Strasbourg, France, 1998–2010, 6 RATS vs. 15 sternotomies (22)].
A more recent report of four European thoracic surgery centers between 2002 and 2011 reported on 79 patients (University of Berlin, Germany, 39 patients; University of Padova, Italy, 14 patients; University of Pisa, Italy, 13 patients; University of Innsbruck, Austria, 13 patients) undergoing RATS on patients with early stage thymomas (size range 1–12 cm) that were operated by a left sided (82.4%), right-sided (12.6%), or bilateral approach (23). Fifty-seven percent of the patients were diagnosed with myasthenia gravis. Operating times ranged from 70–320 minutes. Only one patient required conversion for an oncological reason (large tumor). Hospital stay ranged from 2–15 days. Postoperative complications were reported in 12.7% of patients (none of the complications required additional surgery.
A large series on patients with myasthenia gravis undergoing extended thymectomy compared 79 VATS thymectomies [1994–2002] with 74 RATS thymectomies [2003–2006] at the University of Berlin. Improved outcome in patients with myasthenia gravis who received a RATS Thymectomy compared with the previously used VATS approach was observed. After a follow-up of 42 months cumulative complete remission rates of myasthenia gravis for RATS and VATS thymectomy were reported as 39.2% and 20.3% (P=0.01), respectively (24).
At present it seems that the robotic approach is gaining widespread acceptance in thymic and mediastinal surgery. It is the preferred approach in our own practice at the Medical University Vienna and many other European institutions (25). After a short learning curve of 15 RATS thymectomies (26) similar operating times and improved radicality can be achieved compared with VATS approaches (24).
A left sided (18,27) and a right sided (28) approach were described. In a recent multi-institutional report the left-sided approach was employed in 38%, the right-sided in 59.8% and a bilateral approach in 2.2% of patients (28). Even few of these centers started with a right-sided approach; there is a trend towards using a left-sided approach in European practice. Our own video material showing the dissection of the left phrenic nerve (Figure 1), thymic horns (Figure 2), and the thymus on the right side (Figure 3) via our left sided three-port RATS approach are available on the journal’s website.



Subxyphoid approach
The benefits of subxiphoid video-assisted thoracoscopic thymectomy for thymoma were recently described [2015, Pulmonary Hospital, Zakopane, Poland (32)]. The subxiphoid approach provides excellent view of the anterior mediastinal space, the right phrenic nerve and both costophrenic angles. The avoidance of entering intercostal spaces may be responsible for minimal pain after closure. Proponents of the “maximal thymectomy” technique described a modification of the subxyphoidal procedure to improve exposure in the cervical region. The resection of Masaoka stage I-III thymomas with the use of minimally-invasive extended thymectomy performed through the subxiphoid-right or bilateral VATS approach with double elevation of the sternum allows visualization of the lower poles of the thyroid [2009–2012, 14 patients, Pulmonary Hospital, Zakopane, Poland (33)].
Combined approaches
Transcervical-subxiphoid-VATS maximal thymectomy is probably the MIS equivalent to maximal thymectomy avoiding sternotomy in patients with myasthenia gravis. The operation involves several incisions: cervical, subxiphoid and two for VATS. The cervical part of the procedure is performed with an open technique, the intrathoracic part of the procedure is performed with the videothoracoscopy assisted (VATS) technique [Pulmonary Hospital, Zakopane, Poland (34)].
A minimally invasive subxiphoid-right, videothoracoscopic technique was not only described for thymectomy of thymoma but also for rethymectomy [Pulmonary Hospital, Zakopane, Poland (35)].
We want to report our first experiences with two cases of VATS left-subxiphoid extended thymectomy for the resection of thymomas >5 cm in diameter in Masaoka-Koga stage II. This approach provides excellent control of the left phrenic nerve and innominate vein at the time of left-sided VATS dissection while the right cardiophrenic fatty tissue and right phrenic nerve can be easily controlled through the subxyphoid incision. The operative specimen containing large thymomas can be retrieved from the subxiphoid incision. There was no necessity for sterna lifting (submitted for publication).
Conclusions
As one would expect there is no single European approach to thymic MIS. First reports of thymic surgery for thymic diseases date back to the German surgeon Sauerbruch. Surgeons from the European continent have helped shape thymic surgery together with valuable contributions from all over the globe to its current form. There is no reason to believe that the near future will not bring even more achievements in surgical techniques and technical innovations to thymic surgery and that a unified surgical approach to the thymus may not become a reality. The large repertoire of MIS techniques in the hands of experts harbors one perfect approach for every patient qualifying for thymic MIS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ruffini E, Venuta F. Management of thymic tumors: a European perspective. J Thorac Dis 2014;6 Suppl 2:S228-37. [PubMed]
- Detterbeck FC. The international thymic malignancy interest group. J Natl Compr Canc Netw 2013;11:589-93. [Crossref] [PubMed]
- Okumura M. Trends and current status of general thoracic surgery in Japan revealed by review of nationwide databases. J Thorac Dis 2016;8:S589-95. [Crossref] [PubMed]
- Falcoz PE, Brunelli A. The European general thoracic surgery database project. J Thorac Dis 2014;6 Suppl 2:S272-5. [PubMed]
- ESTS Database annual report 2015. Available online: http://www.ests.org/_userfiles/pages/files/ESTS%20201Silver_Book_FULL_PEF.pdf, accessed 2 March 2017.
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol 2014;9:541-8. [Crossref] [PubMed]
- Crile G Jr. Thymectomy through the neck. Surgery 1966;59:213-5. [PubMed]
- Jaretzki A 3rd, Penn AS, Younger DS, et al. "Maximal" thymectomy for myasthenia gravis. Results. J Thorac Cardiovasc Surg 1988;95:747-57. [PubMed]
- Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Ruffini E, Guerrera F, Filosso PL, et al. Extended transcervical thymectomy with partial upper sternotomy: results in non-thymomatous patients with myasthenia gravis. Eur J Cardiothorac Surg 2015;48:448-54. [Crossref] [PubMed]
- Zieliński M. Rethymectomy in the Treatment of Refractory Myasthenia Gravis: Operative Technique Through the Transcervical Approach. In: Zieliński M, Rami-Porta R. editors. The Transcervical Approach in Thoracic Surgery. Berlin, Heidelberg: Springer Berlin Heidelberg, 2014:137-40.
- Mineo TC, Pompeo E, Lerut TE, et al. Thoracoscopic thymectomy in autoimmune myasthesia: results of left-sided approach. Ann Thorac Surg 2000;69:1537-41. [Crossref] [PubMed]
- Popescu I, Tomulescu V, Ion V, et al. Thymectomy by thoracoscopic approach in myasthenia gravis. Surg Endosc 2002;16:679-84. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Adjuvant pneumomediastinum in thoracoscopic thymectomy for myasthenia gravis. Ann Thorac Surg 1996;62:1210-2. [Crossref] [PubMed]
- Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg 2014;45:e187-93. [Crossref] [PubMed]
- Toker A. Standardized definitions and policies of minimally invasive thymoma resection. Ann Cardiothorac Surg 2015;4:535-9. [PubMed]
- Dunning J. Video-assisted thoracoscopic microthymectomy. Ann Cardiothorac Surg 2015;4:550-5. [PubMed]
- Rea F, Marulli G, Bortolotti L, et al. Experience with the "da Vinci" robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann Thorac Surg 2006;81:455-9. [Crossref] [PubMed]
- Rückert JC, Ismail M, Swierzy M, et al. Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann N Y Acad Sci 2008;1132:329-35. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [Crossref] [PubMed]
- Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. [Crossref] [PubMed]
- Renaud S, Santelmo N, Renaud M, et al. Robotic-assisted thymectomy with Da Vinci II versus sternotomy in the surgical treatment of non-thymomatous myasthenia gravis: early results. Rev Neurol (Paris) 2013;169:30-6. [Crossref] [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Rueckert J, Swierzy M, Badakhshi H, et al. Robotic-assisted thymectomy: surgical procedure and results. Thorac Cardiovasc Surg 2015;63:194-200. [Crossref] [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [Crossref] [PubMed]
- Marulli G, Maessen J, Melfi F, et al. Multi-institutional European experience of robotic thymectomy for thymoma. Ann Cardiothorac Surg 2016;5:18-25. [PubMed]
- Matilla JR, Klepetko W, Moser B. Dissection of left phrenic nerve in a 29-year-old female patient with myasthenia gravis. Asvide 2017;4:209. Available online: http://www.asvide.com/articles/1519
- Matilla JR, Klepetko W, Moser B. Dissection of the thymic horns in a 22-year-old female patient with myasthenia gravis. Asvide 2017;4:210. Available online: http://www.asvide.com/articles/1520
- Matilla JR, Klepetko W, Moser B. Dissection of the thymus on the right side in a 48-year-old patient with myasthenia gravis. Asvide 2017;4:211. Available online: http://www.asvide.com/articles/1521
- Zieliński M, Rybak M, Wilkojc M, et al. Subxiphoid video-assisted thorascopic thymectomy for thymoma. Ann Cardiothorac Surg 2015;4:564-6. [PubMed]
- Zielinski M, Czajkowski W, Gwozdz P, et al. Resection of thymomas with use of the new minimally-invasive technique of extended thymectomy performed through the subxiphoid-right video-thoracoscopic approach with double elevation of the sternum. Eur J Cardiothorac Surg 2013;44:e113-9; discussion e119.
- Zielinski M, Kuzdzal J, Nabialek T. Transcervical-subxiphoid-VATS "maximal" thymectomy for myasthenia gravis. Multimed Man Cardiothorac Surg 2005;2005:mmcts.2004.000836.
- Zielinski M. Minimally invasive subxiphoid-right, videothoracoscopic technique of thymectomy for thymoma and rethymectomy. Multimed Man Cardiothorac Surg 2012;2012:mms005.
Cite this article as: Matilla JR, Klepetko W, Moser B. Thymic minimally invasive surgery: state of the art across the world—Europe. J Vis Surg 2017;3:70.


