Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy
Introduction
Sub-lobar resection for peripheral small-sized lung nodules such as ground-glass opacities (GGOs) and metastatic pulmonary tumors is useful in obtaining pathological diagnosis and radical cure. Lung segmentectomy is better than wedge resection for securing a sufficient surgical margin. However, anatomical segmentectomy, because of the need to identify variances in pulmonary vessels and the intersegmental line during planning for lung anatomical segmentectomy, is a technically more complicated operative procedure than standard lobectomy. Some surgeons have reported preoperative mapping of pulmonary structures using three-dimensional computed tomography (3D-CT) reconstruction for video-assisted thoracic surgery (VATS) or segmentectomy via thoracotomy (1-4). An understanding of individual variations in the anatomy of pulmonary vessels and bronchi is important for safety, especially when performing anatomical segmentectomy. To identify the intersegmental plane, some surgeons use the inflation-deflation line. Target segmental inflation is the most common method for identifying the intersegmental line (5,6). However, this method is sometimes difficult for patients with emphysema and can be technically unstable if the lung does not deflate sufficiently during the operation. In addition, the inflated lung occasionally obstructs the surgical view during thoracoscopic surgery (TS). Indocyanine green fluorescence (ICGF) has been reported to identify the intersegmental plane without lung inflation (7,8). This report describes our initial experience of 3D-CT imaging and ICGF-navigated thoracoscopic anatomical segmentectomy (TS-S).
Methods
Patients
This study was an exploratory, single-armed study approved by the ethics committee of our institution (No. 2010-1055), and written informed consent was obtained from each patient for publication of this article and any accompanying images. A copy of the written consent is available for review by the Editors-in-Chief of this journal. Our inclusion criterion was planned TS-S for peripheral small-sized lung nodules. Exclusion criteria were allergy to iodine or specific risks related to hepatic or renal function. Twenty patients were performed ICGF-navigated TS-S from January 2013 to May 2014.
Preoperative evaluation
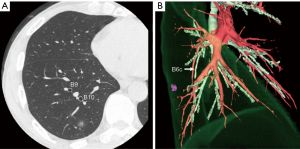
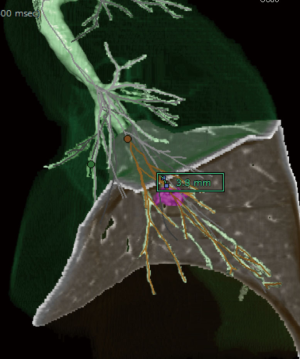
Multidetector-row computed tomography (MDCT) was routinely performed preoperatively (Figure 1A). Using MDCT data, we performed three-dimensional simulation of the vascular structures, segmental bronchus, and tumor using simulation software (Volume Analyzer Synapse Vincent; Fujifilm Medical Systems, Tokyo, Japan; Figure 1B). The simulation workstation allowed us to create a virtual intersegmental plane after plotting the dominant segmental pulmonary artery and to measure the surgical margin from the tumor to the closest intersegmental plane (Figure 2), thus allowing proper preoperative planning for all patients.
Summary of ICG fluorescence
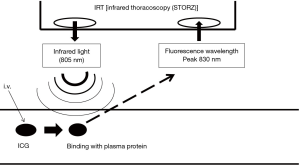
After intravenous injection, ICG binds to plasma protein. If near infrared light (805 nm) is absorbed by the ICG in plasma, it will emit a fluorescence wavelength of 830 nm that can be seen using infrared thoracoscopy (Figure 3). In this study, we used an infrared thoracoscopy system (D-LIGHT P system; KARL STORZ—Endoskope, Japan K.K., Tokyo, Japan). The scope was 10 mm in diameter with a 30-degree rigid scope. The mode of monitoring could be changed instantly between white-light and ICGF modes using a foot switch.
Misaki et al. reported that infrared thoracoscopy with ICG makes it possible to identify the target lung segment easily and quickly without the need for lung inflation (7). In their study, the dominant pulmonary artery of the target segment (TS) was ligated, and after injection of ICG, infrared thoracoscopy revealed ICGF. Their detailed macroscopic and microscopic study confirmed that the demarcation corresponded to the intersegmental line (7,8).
Surgical technique
Although the definition of VATS varies in Japan, in the present study it is defined as an operation performed entirely through four small incisions measuring 25, 15, 7, and 7 mm, with video-monitor visualization only and no rib spreading. In the present study, we refer to our procedure as TS. After dividing the dominant pulmonary arteries and segmental bronchus, ICG was injected into a peripheral vein. Under infrared light, the ICGF line of demarcation became clearly visible. The line was marked using electrocautery, and lung parenchyma was divided using electrocautery or endoscopic staples along this line. As a rule, we tried to keep the surgical margin larger than the size of the tumor.
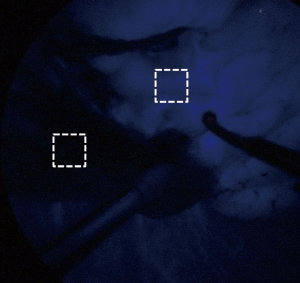
Feasibility and quantitative contrast of ICGF
To assess the feasibility of this technique, all demarcation findings were classified into two categories. A valid temporary demarcation was a clear line along which we had enough time to mark the lung surface. An invalid demarcation was poorly visible or completely invisible. To evaluate the contrast quantitatively, we used image-analysis software (Photoshop Elements 8.0, Adobe Systems Inc., San Jose, CA, USA) to evaluate the strength of luminosity in the TS and non-target segments (NTS) as for a still image (Figure 4). A region of interest measuring approximately 1×1 cm in both the TS and NTS was used to acquire a quantitative value of luminosity. Using the histogram function, we could obtain the average luminosity value (LV) assigned to each pixel from 256 degrees of human-eye sensitivity to color. This procedure was repeated three times, with the median result used as the representative value. The strength of contrast was calculated using the following equation;
Contrast index (CI) = log(LVTS/LVNTS)
where LVTS and LVNTS are acquired LVs for the TS and NTS, respectively. LVTS, LVNTS, and CI were assessed at 30-s intervals after ICGF began.
Results
Baseline characteristics are summarized in Table 1. The study patients comprised 10 men and 10 women (mean age, 65 years; range, 30–87 years). There were 15 primary lung cancers and 5 metastatic lung tumors (3 from osteosarcomas, 1 from colorectal cancer, and 1 from uterine cancer). Preoperatively, 9 patients (45%) had a documented history of smoking. Average pulmonary function as measured by forced expiratory volume in 1 s (FEV1) was 2.25 L (range, 1.28–4.02 L). Mean tumor size was 16 mm (range, 9–32 mm). TS-S, including subsegmentectomies, was complete in all patients. Mean operative duration was 186 min (range, 90–319 min), and the mean blood loss was 30 mL (range, 0–107 mL). There were no intraoperative or postoperative adverse events related to the administration of ICG. All patients were discharged without major complication, but one patient had slight renal dysfunction (Clavien-Dindo grade I). Median duration of chest drainage was 1 day (range, 1–3 days), and the median postoperative hospital stay was 5 days, (range, 5–8 days). The surgical margin was negative for malignancy in all patients; however, in two patients (10%) the tumor margin was less than the size of the tumor because the tumor was located near the trunk of the pulmonary artery. In this situation, even if we had performed a lobectomy, an adequate surgical margin could not have been obtained. The closest surgical margin was not to the intersegmental plane but to the pulmonary artery, so we did not convert to lobectomy.
Full table
The feasibility of ICG fluorescence
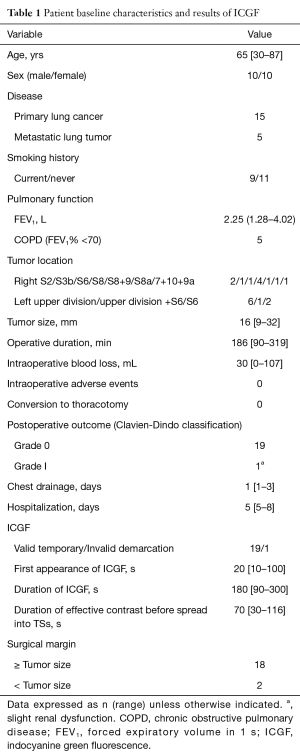
Valid temporary demarcation using ICGF was possible in 19 patients (95%) under the infrared thoracoscopy system. We experienced invalid demarcation in one patient with severe anthracosis on the lung surface. Details of timing, appearance, and duration of ICGF are presented in Table 1. Mean LVNTS decreased over time (Figure 5A), but LVTS slowly increased (Figure 5B). As a result, CI peaked 30 s after the appearance of ICGF and decreased thereafter (Figure 5C). Additionally, 70 s (range, 30–116 s) after ICGF began, there was less effective contrast because of the spread of ICGF into TSs.
Conclusions
We performed TS-S for peripheral small-sized GGO lesions or pulmonary metastases to secure a sufficient surgical margin. However, compared with lobectomy, anatomical segmentectomy is a technically more difficult procedure. When we perform anatomical segmentectomy, after dividing the target segmental pulmonary artery and bronchus, we perform blunt dissection along the intersegmental vein near the hilum and, after identifying the segmental plane, divide the lung parenchyma along the intersegmental line. Variances in pulmonary vessels and bronchus on preoperative MDCT should therefore be carefully evaluated and the intersegmental plane accurately identified intraoperatively. Some authors have reported on preoperative prediction of pulmonary structures for segmentectomy via VATS or thoracotomy (1-4). Saji et al. reported that preoperative and intraoperative guidance by virtual segmentectomy using the Synapse Vincent (Fujifilm Medical Systems) could greatly assist surgeons in achieving the most appropriate anatomical and curative resection (4). We use this workstation with patients undergoing anatomical segmentectomy and plan a surgical procedure that will obtain sufficient surgical margin.
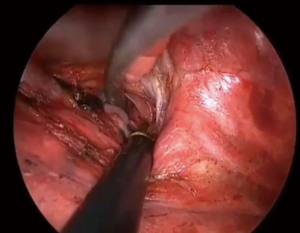
Target segmental inflation is the most common method for identifying the intersegmental plane (5,6) but is sometimes difficult in patients with emphysema. Additionally, the inflated lung can block the operative field of vision during TS. In general, each bronchus is accompanied by a pulmonary artery. Therefore, an intersegmental line can be identified using either the segmental bronchi or the pulmonary artery. Misaki et al. conducted a clinical trial of segmentectomy using an infrared thoracoscopy system with ICG (8). They succeeded in visualizing the differential blood flow of the pulmonary artery in the lung using a prototype infrared thoracoscope with injection of ICG 3 mg/kg. Detailed macroscopic and microscopic examination confirmed that the marking corresponded to the intersegmental line. In the present study, the intersegmental line was quickly and clearly visualized using the D-LIGHT P system (KARL STORZ) with injection of a low dose (0.25 mg/kg) of ICG. The scope is 10 mm in diameter with a 30-degree rigid scope and can easily be used for non-fluorescence-guided TS. Additionally, we can record high-definition images in white-light mode and easily change from white-light to ICGF mode using a foot switch. Using this camera system, we can easily create ICGF after intravenous systemic injection of low-dose ICG (0.25 mg/kg) without adverse events. According to other toxicity studies, intravenous injection of up to 5.0 mg/kg ICG is quite safe and acceptable (9). In the present study, the sensitivity of this ICG system was better than that of previous reports, so we were able to create ICGF images twice in one operation (8,10). For example, when we performed right segments (S)9+10 segmentectomy, we first divided the intersegmental line between S6 and the basal segments along the ICGF demarcation after clamping the pulmonary artery of S6. After dividing the segmental arteries and bronchus of S9+10, a second ICGF demarcation could be created for the intersegmental plane between S9+10 and S7+8. Demarcation was clear even in the second ICGF (Figure 6).
TS-S with ICG navigation to maintain a tumor margin was feasible. However, this method has some limitations. First, ICG may be rapidly washed out, resulting in loss of staining. Second, in difficult settings such as severe anthracosis on the lung surface, visualizing a clear contour pattern can be problematic because of hypovascularity on the lung surface. Third, LVTS increases slowly and ICGF spreads into TSs, resulting in a reduced CI. Fourth, although three-dimensional images and intraoperative ICGF demarcation may show that the intersegmental plane is not always flat, the direction of parenchymal dissection using electrocautery or staples during surgery is almost straight.
The feasibility of ICGF despite these limitations is rarely discussed. Tarumi et al. conducted a clinical trial of VATS segmentectomy using infrared thoracoscopy with ICG (10). In that report, identification of the intersegmental line was possible without complication in 11 out of 13 patients (84.6%). Mean operative duration (191 min) and mean blood loss (64 mL) were similar to the values in the other 31 patients, who underwent thoracotomy. The authors concluded that infrared thoracoscopy with ICG was useful for minimally invasive thoracic surgery. In the present study, surgical outcomes were also favorable. Three-dimensional CT imaging using the Synapse Vincent facilitated planning of anatomical segmentectomy to achieve sufficient surgical margins. In addition, ICGF demarcation was easily identified using the segmental pulmonary artery, so TS-S could be safely performed. However, although ICGF could be observed on the lung surface, it could not be seen on the ongoing dissection plane. Additionally, marking the demarcation line with electrocautery had to be done carefully because the background is dark in ICG mode.
In this study, CI decreased and ICGF spread to TSs. The factors influencing these occurrences are not clear because our sample size was small. The parenchyma may be supplied not only by pulmonary artery, but also by vessels including the bronchial artery. Further investigation with larger-sized study samples may elucidate this.
In conclusion, intraoperative ICG-fluorescence navigation was applied safely and successfully to TS-S. Large prospective studies are needed to investigate further the factors influencing ICGF CI, oncological benefits, and long-term outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was an exploratory, single-armed study approved by the ethics committee of our institution (No. 2010-1055), and written informed consent was obtained from each patient for publication of this article and any accompanying images.
References
- Fukuhara K, Akashi A, Nakane S, et al. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg 2008;34:875-7. [Crossref] [PubMed]
- Oizumi H, Endoh M, Takeda S, et al. Anatomical lung segmentectomy simulated by computed tomographic angiography. Ann Thorac Surg 2010;90:1382-3. [Crossref] [PubMed]
- Shimizu K, Nakano T, Kamiyoshihara M, et al. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact Cardiovasc Thorac Surg 2012;15:194-6. [Crossref] [PubMed]
- Saji H, Inoue T, Kato Y, et al. Virtual segmentectomy based on high-quality three-dimensional lung modelling from computed tomography images. Interact Cardiovasc Thorac Surg 2013;17:227-32. [Crossref] [PubMed]
- Atkins BZ, Harpole DH Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 1112-3. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. [Crossref] [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Fox IJ, Wood EH. Indocyanine green: physical and physiologic properties. Proc Staff Meet Mayo Clin 1960;35:732-44. [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
Cite this article as: Mun M, Okumura S, Nakao M, Matsuura Y, Nakagawa K. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017;3:80.


