Video assisted thoracoscopic and open chest surgery in diagnosis and treatment of malignant pleural diseases
Introduction
There are three different mechanisms by which the pleura can be involved by malignancy: primary tumour, extension from adjacent tumour or pleural metastases (via haematogenous or lymphatic spread). The disease can manifest either with a solid or fluid component, or a combination of the two. Regardless of the causing pathology and histopathologic form, malignant pleural disease is normally associated with poor prognosis (1,2).
Patients with intrathoracic or extrathoracic malignancies complicated by malignant pleural effusions have a median survival of 4 months (2). Parenchymal cancers of lung, breast, gastrointestinal tract and ovaries as well as lymphomas and mesotheliomas are among the most common cancer types causing malignant effusions, however near all tumour types have been described to cause a malignant effusion (3-8).
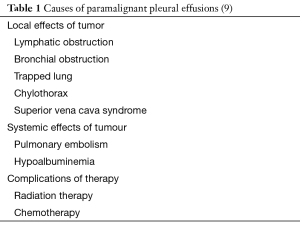
The incidence of malignant and paramalignant effusions in patients with metastatic malignancies can be as high as up to 50% (2). Paramalignant pleural effusions result from mechanical effects caused by the tumours to the pleural space (airway obstruction, mediastinal lymph node involvement, superior vena cava syndrome) while pleural fluid cytology and biopsies may remain non-diagnostic (Table 1). Histopathology results from fluid cytology or pleural biopsies are positive for cancer when a malignant effusion is present (9). It has been suggested from studies contacted post-mortem, that pleural metastases can occur due to tumour emboli on the visceral pleura which may result to parietal pleural seeding (10,11). Direct spread via neighbouring tumours as well as indirect spread via blood or lymphatic streams may also occur. When a topical inflammatory process develops due to tumour invasion, it could potentially lead to increased capillary permeability and development of effusions (12).
The prognosis heavily depends on patients’ response to systemic therapy. To date, there are not sufficient data to allow accurate predictions of survival that would facilitate decision making for managing patients with malignant pleural diseases. Management in most cases remains palliative; it should be stressed however that the appropriate management approach should be based on available treatment options and medical expertise as well as the patient’s clinical status. Malignant pleural effusions can severely impair patients’ quality of life. Multiple palliative approaches are available to drain the effusion, and to prevent it from accumulating, thus providing adequate symptomatic relief. Asymptomatic patients with malignant pleural effusions do not normally require treatment. Enrolment of patients in clinical trials, when these are available, is imperative for standardisation of different approaches, as well as utilisation of multi-modality and multi-level treatments that would provide the best possible outcome. Time is one of the few privileges we can provide to these patients; and we should aim to make every second count, taking good care not to sacrifice the quality of their remaining life in exchange.
Diagnosis
Dyspnoea remains the commonest presenting symptom in patients with malignant effusions. Patients may also present with non-specific symptoms, such as loss of appetite, loss of weight and fatigue, depending on the stage of their disease. More specific symptoms, including localised chest pain, cough and haemoptysis, are normally associated with distinct pathologies such as mesothelioma or bronchogenic carcinoma (13). Patients with a malignant effusion due to sarcoma have been reported to present with a pneumothorax (14).
Pleural effusions holding at least 50 mL of fluid can be visualised on lateral chest films thus initiating more detailed investigations (15). Other radiographic signs include crowded ribs, elevated hemidiaphragm, pleural thickening, lung atelectasis and ipsilateral mediastinal shift (16).
Thoracic ultrasound can be also used to confirm fluid collections, assess their characteristics and guide intervention (17,18). When morphological criteria similar to contrast-enhanced CT are applied, thoracic ultrasound can differentiate malignant and benign effusions with an estimated sensitivity of 79% and specificity of 100% (19).
Computed tomography with contrast enhancement provides the most useful information for the evaluation of patients with suspected malignant effusions, while also allowing for the detection of associated intra or extrathoracic disease (lymphadenopathy, parenchymal/bone lesions). CT thorax can differentiate benign from malignant disease by identifying specific characteristics, such as pleural thickening (20,21). In the case of pleural mesothelioma differentiation from metastatic pleural malignancy can be more challenging, as the two conditions share many CT features. Characteristics indicative of mesothelioma include involvement of the interlobar fissures, pleural thickening >1 cm and presence of calcified pleural plaques (22).
Magnetic resonance imaging is not routinely used in the investigation of pleural effusions. It can however prove useful in occasions when use of contrast agents is contraindicated as well as for the assessment of diaphragmatic and chest wall involvement, where it has been confirmed to have superior accuracy when compared to computed tomography (23).
Positron emission tomography for malignant pleural disease has a reported sensitivity of 93% to 100% and specificity of 67% to 89% (24). False-positive results can occur in patients with accompanying inflammatory pleural conditions or following interventions, such as talc pleurodesis (25). Fused images can be used to guide diagnostic biopsies and differentiate between activity due to talc pleurodesis, by detecting pleural thickening with increased CT attenuation (26).
Principles of management of malignant effusions of the pleura
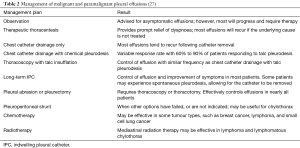
As prognosis remains poor, management of malignant pleural effusions is primarily palliative and aims to provide effusion control, allowing for symptomatic relief (Table 2). Early interventions are strongly advocated by some centres, in order to reduce future complications by preventing development of pleural loculations and infected cavities.
Interventions are directed towards drainage of the effusion and, when appropriate, concurrent or subsequent pleurodesis is performed to prevent fluid re-accumulation. Alternatively, permanent or semi-permanent drainage may be established for long-term management of recurrences. An initial thoracentesis does not decrease the effectiveness of subsequent procedures to produce pleurodesis. The appropriate management plan is devised based on individual patient characteristics such as the rate of re-accumulation, disease prognosis, and severity of symptoms.
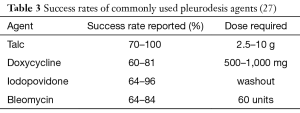
Chemical pleurodesis has been widely adopted for means of palliation in patients troubled by symptomatic and recurrent effusions; a great selection of chemicals can be used, employed via different techniques, in an attempt to produce pleurodesis (Table 3). To this date, it remains somewhat uncertain if one agent is superior to another (28) however talc pleurodesis remains the preferred choice in many centres (29).
Management of malignant pleural effusions in specific diseases
Metastatic carcinoma
Lung
Lung cancer is the primary cause of malignant pleural effusions. During the course of the disease, the probability of such effusions to manifest can reach 35% (30). Even though effusions can occur with any histologic type, adenocarcinoma is the most frequent type observed (13,31). Presence of pleural effusion usually indicates an advanced stage of the disease (32), however further investigations should be carried out to confirm or exclude pleural involvement, as the effusion could be due to mechanical obstruction or imbalance of regional lymphatics and not associated with direct pleural disease (33). When non-invasive diagnostic techniques are unable to yield diagnosis, video assisted thoracoscopic surgery (VATS) is indicated. Not only it allows direct visualisation of the entire thoracic cavity and facilitates obtaining pleural biopsies from multiple locations, but also offers the option of providing concomitant treatment by achieving lung re-expansion and performing pleurodesis procedures, in a more efficient manner (34).
In metastatic pleural disease, unfavourable prognosis means that longer recovery periods from majorly invasive procedures are not justified. Surgery should be offered for diagnosis and palliation only, thus a minimally invasive technique such as VATS instead of open thoracotomy should be the only surgical approach to be considered (35).
Breast
Breast carcinoma ranks as the second most frequent cause of malignant pleural effusion. During the course of the disease, the probability of such effusions to manifest can be as high as 23% (30). Breast cancer patients can have different degrees of invasion (36) and can present with unilateral, ipsilateral or bilateral effusions (37,38). Further investigations for exclusion of non-malignant effusions should always be considered in patients who underwent postoperative chemotherapy (39). These effusions respond well to conservative management, whereas surgical management should be directed towards symptomatic relief and prevention of recurrence. Median survival will depend on response to systematic therapy (40), therefore it is vital that these patients can commence their treatment at earliest possible, without having to go through long recovery periods from their operations. Staged VATS procedures may be required for management of bilateral malignant effusions.
Hematopoietic or lymphoid malignancies
Lymphoma
Lymphomas are the cause of 10% of malignant pleural effusions (41). In Hodgkin’s lymphoma pleural effusion develops in the later stages of the disease, while in non-Hodgkin’s lymphoma effusion can be seen as early as the time of diagnosis (42). The effusion may be unilateral or bilateral. The enlarged mediastinal lymph nodes are responsible for the obstruction of lymphatic flow in Hodgkin’s disease, while in non-Hodgkin’s lymphoma this is caused by direct infiltration of the parietal and/or visceral pleurae by tumour (43). Chylothorax most commonly manifests in non-Hodgkin’s lymphomas (44).
Systematic chemotherapy is the treatment of choice while mediastinal radiotherapy is also given in cases of mediastinal involvement (44). If chylothorax occurs, conservative management is usually recommended as first line management, which consists of tube thoracostomy drainage, combined with low fat, medium-chain triglyceride supplemented regimens, or total parenteral nutrition in an attempt to reduce recurrence (45,46).
Surgical thoracoscopy is reserved for refractory chylothorax, which permits adequate drainage of the thoracic cavity and allows for concurrent pleurodesis to be performed, while it also facilitates ligation of the thoracic duct when indicated (47-50). Pleuroperitoneal shunt may also be considered when other measures fail to control reaccumulation of chyle (51). Although effective palliation has been reported in approximately 70–100% of patients, the incidence of shunt occlusion is as high as 25% at a median time of 2.5 months (29). Furthermore, the shunts can apply further burden on the patients, as they have to be frequently pumped during the course of a day.
Myeloma and leukaemia
Pleural effusions develop in about 6% of patients with multiple myeloma, of which less than 1% are classified as malignant (52). They reflects poor prognosis, with mean survival of less than 4 months (53). Suspected mechanisms include extension of plasmacytomas of the chest wall, invasion from adjacent skeletal lesions, direct pleural involvement by myeloma or following lymphatic obstruction secondary to lymph node infiltration (54).
In patients with chronic myeloid leukaemia pleural effusion is rare, and hasn’t been thoroughly examined (55). The possible mechanisms of exudative pleural effusion include leukemic infiltration into the pleura, extramedullary haemopoiesis, possible obstruction of pleural capillaries or infiltration of interstitial tissue by leukemic cells, increased capillary permeability, non-malignant causes (infection, hypoproteinemia) and drugs (56).
Systemic treatment is indicated; surgical management is restricted to diagnostic and pleurodesis procedures.
Malignant pleural mesothelioma
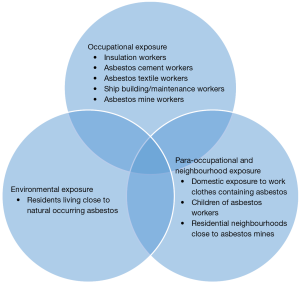
Less than 1% of all new cancer cases in the UK are diagnosed with mesothelioma. In 2014 alone 2,700 new cases were diagnosed with the disease, which equals more than 7 new cases every day. The disease predominantly affects males and half of the newly diagnosed cases are individuals aged 75 and over. One in every hundred men born in 1940s are estimated to die because of it. Mesothelioma incidence rates in Great Britain have increased almost six-fold since the late 1970s and by around a tenth over the last decade, with a larger increase in females than males. Incidence rates for mesothelioma in the UK are projected to fall by 53% between 2014 and 2035, to 3 cases per 100,000 people by 2035. Approximately 2,100 people had survived the disease in the UK at the end of 2006, ten years after they were diagnosed with mesothelioma. Asbestos has been linked with approximately 94% of mesothelioma cases in the UK and remains the main potentially avoidable risk, while other risk factors may be related however they haven’t been as extensively studied (Cancer Research UK: https://www.cancerresearchuk.org/) (Figure 1).
The global incidence of mesothelioma is not easy to estimate, mainly because it is a relatively rare cancer and not reported by many developing countries. Based on a combination of mortality data and asbestos use, an average of 14,200 mesothelioma cases are diagnosed worldwide each year. The highest number of cases in the world in encountered in the United States and the UK. In most European countries, the increase in incidence slowed down or remained static between the late 1980s and mid 1990s however, production and use of asbestos continues in many parts of the world, with Russia and China being the main exporters (Cancer Research UK: https://www.cancerresearchuk.org/).
In contemporary medicine, not many cancers have managed to generate as intense debates regarding treatment as does malignant pleural mesothelioma. The relative advantages of surgery, radiation, chemotherapy and any combination of the three, are continuously reassessed and reconsidered, even though not always based on scientific evidence. The average life expectancy for mesothelioma patients ranges from 12 to 21 months; about 40% of mesothelioma patients survive the first year following diagnosis, and 20% live more than 2 years. There’s great variation between individual reports which is unsurprising, considering the long incubation period and the often-late diagnosis of the disease. Today patients live longer than ever before, some survive 3, 5, even 10 years (American Cancer Society: https://www.cancer.org) (Asbestos.com: www.asbestos.com).
The aim of surgery in mesothelioma may be prolongation of life, in addition to palliation of symptoms. Longer recovery periods from more extensive surgical procedures could be justified, in carefully selected patients. Surgical options include: VATS pleurodesis, VATS partial pleurectomy (VATS PP)—both parietal and visceral; open pleurectomy decortication (PD)—with an extended option (EPD) and extrapleural pneumonectomy (EPP).
Diagnostic and palliative surgical procedures
Most patients during the time of their presentation will be found to be in an advanced stage of the disease, and there are currently not enough data to support that a radical surgical approach would be of benefit, given the risks of the operation and the long recovery period. In these end-stage scenarios surgery might be performed with a palliative intent, aiming to reduce the amount of fluid in the pleural cavity and allow lung re-expansion. Palliative surgical procedures should be primarily minimally invasive, to reduce the potential harmful effects of a thoracotomy (58).
Thoracoscopy for biopsy and pleurodesis
“Medical thoracoscopy” can be performed with a rigid bronchoscope under local or regional anaesthesia. The procedure can be performed for diagnosis or treatment, when it involves talc pleurodesis. A study by Valsecchi et al., that included 2,752 patients who received medical thoracoscopy between 1984 and 2013, found that the overall likelihood that medical thoracoscopy would provide the information needed to accurately diagnose lung diseases such as mesothelioma increased from 57% to nearly 80% over the course of the study period. Mesothelioma patients who presented with a pleural effusion, had a greater diagnostic yield than patients without an effusion (59).
VATS biopsy and pleurodesis is generally performed under general anaesthesia with double lumen intubation. It can also be accomplished with single lumen intubation or sedation and local anaesthesia (60). The patient is placed on the operating table in lateral decubitus position and the chest is prepped and draped as it would for a thoracotomy. Following lung isolation, the camera and the instruments are inserted in the thoracic cavity, preferably via a single, limited incision below the tip of the scapula, in the line of a future thoracotomy incision [thus limiting the possibility of disease dissemination via the wound (61)]. A small open incision may be performed in the case of a “dry” presentation of the disease when a complete obliteration of the pleural space precludes thoracoscopy. Detailed exploration of the thoracic cavity is performed after drainage of the effusion and biopsies are obtained from the anterior, posterior and diaphragmatic pleura. Deep and large biopsies, preferably including fat and/or muscle should be taken to enable assessment of possible tumour invasion. The anaesthetist is asked to re-inflate the lung, and its ability to fully re-expand and approximate the chest wall is evaluated. When the lung is seen to fully re-expand, asbestos-free talc is insufflated in the chest taking caution to distribute the powder to cover the entire cavity. After talc has been administered, a chest tube is positioned and remains in situ on mild suction, for a period of at least 48 hours, to allow the inflammatory process to take place and the lung to attach to the chest wall. The pleural space will then become sealed with scar tissue and fluid won’t be able to re-accumulate. Talc pleurodesis has been found to achieve control of symptoms and low rate of recurrence (62,63). It has also been reported that a complete and persistent lung expansion after the procedure leads to a better prognosis (64). Povidone iodine can also be used in substitute of talc (65), especially if there’s a suspicion for an infection complicating the effusion.
Chemical pleurodesis may be effective only when pleural apposition can be achieved, which depends on the ability of the underlying lung to fully re-inflate. Visceral pleura involvement by the tumour will normally result to an entrapped lung. In these cases, insertion of a permanent drainage catheter (66) or the risks and benefits of more extensive surgery, will need to be considered (67).
Both medical thoracoscopy and VATS are safely acceptable, with low mortality rates reported in the literature (68). VATS positive predictive value has been reported to be as high as 99.7% (69). Compared to medical thoracoscopy, VATS allows for a more efficient drainage of loculated effusions trapped in dense fibrous bands. Medical thoracoscopy can be a cost-effective procedure in patients with poor tolerance for general anaesthesia, in an outpatient setting. Ideally, every case should be discussed between surgeons and interventional pulmonologists in a multidisciplinary setting. The TAPPS trial is currently underway to aid with decision making.
Thoracoscopic debulking: partial pleurectomy/decortication
More advanced stages of the disease can result to an entrapped lung and parietal pleurectomy debulking may be considered as an addition to talc pleurodesis (79). It may be carried out effectively by VATS, achieving 90% effusion control at 12 months (80). The pleural space can be effectively obliterated by successful lung mobilisation combined with pleurectomy to lower the burden of the disease (81).
VATS PP can be performed via one or more ports. Parietal pleurectomy is performed by developing the extrapleural plane using thoracoscopic or traditional instruments, working down to the diaphragm and over the apex. The dissection plane is extended to the upper mediastinum, if possible. It is not normally possible to extend the dissection of the parietal pleura off the pericardium and the central portion of the diaphragm. Lung re-expansion is assessed and if it has not been achieved following fluid drainage, the anaesthetist is asked to apply positive pressure ventilation and then sharp and blunt dissection of the visceral pleura is undertaken in order to release the trapped lung. After securing haemostasis, apical and basal intercostal drains are routinely inserted and initially managed with mild suction. Their stay is guided by air leak. Systematic lymph node dissection is not routinely undertaken as the results of it are not expected to alter further management, which will involve systematic therapy.
A best evidence topic by Srivastava et al. reviewed five prospective cohort studies to examine whether VATS decortication improved prognosis in patients with advanced malignant mesothelioma. The study concluded that VATS provides a diagnostic tool, while drainage of effusion and pleurectomy/decortication improves the quality of life and may also increase survival. However, definitive conclusion could not be drawn, thus a trial was designed in an attempt to give an answer to the question.
Radical surgical procedures
Cancer-directed surgery has been independently associated with better survival (84), however substantial controversy remains as to what should be the recommended treatment strategy for malignant pleural mesothelioma.
Several studies contacted over the years have shown that patients with favourable disease characteristics may benefit from surgery with curative intent, in the context of multimodality therapy (85). Non-epithelioid histology, poor performance status (PS), low haemoglobin, male gender, high platelet count, high lactate dehydrogenase and high white blood cell count have been identified as poor prognostic indicators in mesothelioma (86). In a study by J. Francart et al., the same variables, except for gender, were found to be significant for progression free survival (87). At multivariate analysis of the SEER study, independent significant predictors of survival were female gender, disease stage (distant versus local disease) and age. Survival was improved in the most recent calendar year of diagnosis, for patients diagnosed in 2005–2009 versus patients diagnosed in 1973–1989. Epithelial histology was associated with best survival in comparison to the other histologic types (84).
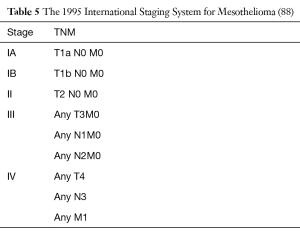
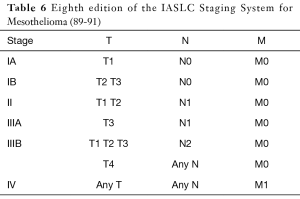
A widely acceptable staging system for malignant pleural mesothelioma had been practically non-existent for more than 40 years. A TNM staging system based on outcomes of retrospective surgical series and limited clinical trials was proposed by the International Mesothelioma Interest Group (iMig) in collaboration with the International Association for the Study of Lung Cancer (IASLC) in 1994 and was accepted by the American Joint Commission on Cancer and the Union for International Cancer Control. Since then it’s considered the international staging standard (Tables 4,5).
More recently, the IASLC Staging Committee, developed an international database for the first evidence-based revision of the TNM staging system, and the eighth edition of the TNM classification for pleural mesothelioma is currently underway. According to revisions of TNM descriptors, both clinical and pathological T1a and T1b are expected to collapse to a single T1 classification (89), both clinical and pN1 and pN2 categories should fall into a single N category comprising ipsilateral, intrathoracic nodal metastases (N1) and nodes previously categorized as N3 should be reclassified as N2 (90). No changes were proposed for M descriptors (91) (Table 6).
EPP
EPP is a highly complex intervention during which the parietal and visceral pleurae are resected en bloc with ipsilateral lung, hemidiaphragm and pericardium and has been associated with significant postoperative morbidity and mortality (92). Because locoregional recurrence comprises a major barrier to long-term survival, adjuvant radiotherapy has been advocated for local disease control; the removal of the lung in EPP allows for high-dose radiation without the risk of causing radiation pneumonitis. However, the routine use of hemithoracic radiotherapy was not shown to provide significant survival benefit according to the results of a recent randomised, international, multicentre phase 2 trial (93). Nonetheless, EPP has been combined with a range of other neoadjuvant and adjuvant strategies, including systematic and intrapleural chemotherapy and photodynamic therapy, aiming to improve OS (67,94).
Procedure technique
An epidural catheter is placed before the operation for postoperative pain control. The patient is then placed in lateral decubitus position and following double lumen intubation and single lung ventilation, an extended s-shaped posterolateral thoracotomy incision is performed, including when possible any previous incisions performed for diagnostic and pleurodesis purposes. Incisions that cannot be included in the thoracotomy incision should be excised separately at the end of the procedure unless the patient is expected to receive radiotherapy post-op. The thoracotomy incision can be extended further, towards the costal margin, providing exposure for diaphragmatic resection and reconstruction when necessary. The sixth rib is divided posteriorly to allow better access into the thoracic cavity. A median sternotomy approach has also been reported in selected cases with no chest wall involvement and low burden of disease as an alternative approach that would result in better pain control and speedier post-operative recovery (101).
An extrapleural plane is created by using blunt dissection, with sweeping motion of fingers, to separate the tumour from the endothoracic fascia. The dissection continues up to the apex, then down to the diaphragm, anteriorly to the pericardium, and posteriorly to the spine. Special care should be applied when dissecting near the azygous vein on the right and the aorta on the left, as dissection may lead to avulsion of the azygous vein or intercostal branches off the aorta. On the left side, attention is taken to identify the plane between the tumour and the adventitia of the aorta and the oesophagus. A nasogastric tube is positioned prior to dissection in order to aid in identifying the oesophagus; the tube can be kept in situ for gastric decompression, to minimize the risk of aspiration in the early postoperative period. Alternatively an oesophageal bougie can also be positioned, as its rigid shape makes it easier to identify. Caution should also be applied when dissecting apically to avoid injury to the subclavian vessels, as well as when dissecting near the superior vena cava on the right. Anteriorly, the plane is continued until the border of the thymic fat and the pericardium. The anterior pericardium is incised and the inner surface is inspected for evidence of invasion, particularly in cases of substantial pleural effusion. The pericardium is resected en bloc with the anterior mediastinal pleura.
Inferiorly the diaphragmatic tumour is resected, taking care to identify the phrenic veins draining from the diaphragm directly into the inferior vena cava on the right. On the left a rim of diaphragmatic crus is preserved, to minimize risk of gastric herniation. The plane between tumour and normal diaphragmatic muscle or peritoneum at the level of the costophrenic sulcus can be developed to free the tumour from the diaphragm, however when involvement of the diaphragm is suspected it should be removed entirely to ensure macroscopic clearance margins.
The sequence in which the hilar structures are divided is decided by their exposure. The superior and inferior veins are divided intrapericardially. Although the right pulmonary artery is usually divided intrapericardially, the left is generally divided extrapericardially, due to its short intrapericardial length. The mainstem bronchus is dissected free from lymph nodes, divided and closed, and the bronchial stamp is tested for air leak. An intercostal muscle flap or similar strategies can be applied at the end of the procedure, to minimise risk of a bronchopleural fistula. The specimen consisting of pleura, lung and diaphragm, with or without pericardium, is removed en-bloc. Systematic lymph node dissection is undertaken for staging purposes.
The diaphragm is reconstructed with a Gore-tex® patch. An absorbable mesh is used for pericardial reconstruction, to prevent cardiac herniation and facilitate postoperative radiotherapy. The pericardial patch must be loose with adequate fenestrations to minimize risk of tamponade, but secure enough to prevent herniation. The inferior border of the pericardial patch must be secured to the diaphragmatic patch in addition to the pericardial rim, to prevent herniation of abdominal contents medially.
A large-bore chest tube, usually 32 Fr, is positioned near the diaphragmatic patch to allow for monitoring of bleeding and/or stump breakdown. The thoracotomy incision is closed and the intercostal muscles are re-approximated, to prevent fluid draining through the wound from the pleural space.
Interestingly, a case of video-assisted thoracoscopic EPP has also been reported (102).
Complications
Patients after their operation should be transferred to a highly monitored environment, such as an intensive care or high dependency unit. The first days of the operation are crucial to the recovery period, as immediate post-op complications should be recognised and addressed promptly. The extensive dissection could result in injuries to endothoracic structures, causing bleeding, vocal cord paralysis, hypotension, arrhythmias, increased risk of aspiration, chylothorax, herniation, thromboembolic events, fistulae, sepsis and death (103).
EPD
Even before the publication of the MARS trial results, a transition from EPP to EPD was already underway due to the shift in demographics of mesothelioma patients, the strict selection criteria applied for EPP, the reported risks and unverified benefits associated with the procedure (104). The transition from EPP to EPD, primarily enabled surgeons to operate on patients that would be denied a pneumonectomy because of age or frailty, without significantly affecting hospital resources and OS (105).
Current evidence suggests that EPD should be preferred when technically feasible, as it is associated with a 2 ½-fold lower short-term mortality than EPP (106). Even though it should be emphasized that patient selection and treatment strategies are different between EPP and EPD, a meta-analysis found that when EPD was performed in a selected group of patients, it resulted to lower perioperative morbidity and mortality with comparable, if not superior, long-term survival compared to EPP, in the context of multi-modality therapy (107).
Procedure technique and complications
The procedure follows similar steps with an extrapleural pleurectomy. The lung is preserved with only non-anatomical resections being performed when necessary, if parenchymal involvement through the visceral pleura is evidenced or suspected intraoperatively. After the parietal pleura has been fully mobilized, it is incised to create a plane into the pleural space. The underlying lung is stripped away from parietal pleura, also removing all areas of visceral pleural tumour. Talc pleurodesis which normally proceeds open radical surgery will have caused a thick fibrotic reaction, fusing pleurae together, and that can provide substantial aid during visceral decortication. In patients with extensive visceral pleural disease, this part of the dissection may require a considerable amount of time and is expected to result to an extensively or completely denuded lung. The difficulty is depending on the frequency of septae which interrupt the smooth parenchyma of the lung surface, creating lobules that make visceral pleurectomy technically challenging. Intriguingly, removal of the tumour from the pulmonary fissures can be completed swiftly as the number of septae present in the fissure is almost always minimal.
As per the definition of the procedure (111), diaphragmatic and/or pericardial resection might be required to secure macroscopic clearance of the disease. During diaphragmatic reconstruction, caution should be taken to ensure that the prosthesis will be positioned at the same level as the native diaphragm and will have enough tension to overcome the abdominal pressure, allowing the lung to re-expand to a satisfactory degree. Dehiscence of the diaphragmatic patch can be avoided by placing a nasogastric tube during the time of the operation, which will remain during the first post-operative days relieving any gas that builds in the stomach. Patients can be either slowly re-introduced to oral feeding or parenteral feeding can be given for a short period of days, as per individual surgeon’s preferences.
After complete excision of the tumour from the lung parenchyma, the chest is vigorously irrigated to remove remaining cells. Two or three large-bore chest tubes, usually 32Fr, are positioned at the end of the procedure to ensure adequate air and fluid drainage, allowing for lung re-expansion. Fluid output is normally high during the first post-operative days and prolonged air leak is to be expected. Drains should remain in situ until oral feeding is commenced, to identify a potential complication of thoracic duct leakage and chylothorax. Patients can be safely discharged home with a drain once they have recovered to a satisfactory level, with air leak being the only reason requiring medical attention. They can be regularly reviewed as outpatients and their drains can be removed once air leak ceases.
Conclusions
Malignant metastatic disease is associated with a poor prognosis and even though multiple well-tolerated techniques exist, management is intended for control of effusion and palliation.
Malignant pleural mesothelioma is a heterogeneous disease with prognosis determined by cell sub-type and by nodal stage. The selection of the optimal surgical procedure should balance its morbidity with the benefits to the patient. Those with node positive, non-epithelioid disease (bad actors) should receive minimally invasive VATS with the aim of effusion control. Those of better PS with node negative, epithelioid disease should be considered for open surgery but with the specific aim of macroscopic complete resection. Only in the minority with stage I, epithelioid MPM below the median age with PS 0 should be considered, unless proven otherwise.
In all above scenarios, the role of surgery within multimodality therapy should be remembered, as any benefit to surgery in mesothelioma relies heavily on the addition of effective adjuvant therapies. Ongoing research adds to our knowledge; a better genetic and molecular understanding of the disease characteristics may lead to development of modern treatment options, allowing for a successful multimodality therapy in the foreseeable future.
Current evidence implies that (Extended) pleurectomy decortication can be performed reliably in specialised centres with good results, both in terms of mortality and survival; however, no operation has been shown to be beneficial in a prospective randomized controlled clinical trial.
Lastly, when selecting any treatment modality, we need to always remember to weigh into our decisions the patient’s preferences and the effect our actions will impose to the quality of life of the patients themselves, and the family that supports them.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Light RW. Pleural Diseases. Lippincott Williams & Wilkins; 2013: 1.
- Luh SP, Chou MC, Wang LS, et al. Video-assisted thoracoscopic surgery in the treatment of complicated parapneumonic effusions or empyemas: outcome of 234 patients. Chest 2005;127:1427-32. [PubMed]
- Henschke CI, Yankelevitz DF, Davis SD. Pleural diseases: multimodality imaging and clinical management. Curr Probl Diagn Radiol 1991;20:155-81. [Crossref] [PubMed]
- Storey DD. Pleural Effusion. JAMA 1976;236:2183. [Crossref] [PubMed]
- Martínez-Moragón E, Aparicio J, Sanchis J, et al. Malignant pleural effusion: prognostic factors for survival and response to chemical pleurodesis in a series of 120 cases. Respiration 1998;65:108-13. [Crossref] [PubMed]
- Hausheer FH, Yarbro JW. Diagnosis and treatment of malignant pleural effusion. Cancer Metastasis Rev 1987;6:23-40. [Crossref] [PubMed]
- Awasthi A, Gupta N, Srinivasan R, et al. Cytopathological spectrum of unusual malignant pleural effusions at a tertiary care centre in north India. Cytopathology 2007;18:28-32. [Crossref] [PubMed]
- Ismail-Khan R. Malignant pleural mesothelioma: A comprehensive review. Cancer Control 2006;13:255-63. [PubMed]
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Rodrîguez-Panadero F, Borderas Naranjo F, López Mejîas J. Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J 1989;2:366-9. [PubMed]
- Meyer PC. Metastatic carcinoma of the pleura. Thorax 1966;21:437-43. [Crossref] [PubMed]
- Andrews BS, Arora NS, Shadforth MF, et al. The role of immune complexes in the pathogenesis of pleural effusions. Am Rev Respir Dis 1981;124:115-20. [PubMed]
- Chernow B, Sahn SA. Carcinomatous involvement of the pleura. Am J Med 1977;63:695-702. [Crossref] [PubMed]
- Chen W, Shih CS, Wang YT, et al. Angiosarcoma with Pulmonary Metastasis Presenting with Spontaneous Bilateral Pneumothorax in an Elderly Man. J Formos Med Assoc 2006;105:238-41. [Crossref] [PubMed]
- Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: A chest radiograph prediction rule. Academic Radiology 1996;3:103-9. [Crossref] [PubMed]
- Qureshi NR, Gleeson FV. Imaging of Pleural Disease. Clin Chest Med 2006;27:193-213. [Crossref] [PubMed]
- Görg C, Restrepo I, Schwerk WB. Sonography of malignant pleural effusion. Eur Radiol 1997;7:1195-8. [Crossref] [PubMed]
- Yang PC, Luh KT, Chang DB, et al. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol 1992;159:29-33. [Crossref] [PubMed]
- Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009;64:139-43. [Crossref] [PubMed]
- Leung AN, Müller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol 1990;154:487-92. [Crossref] [PubMed]
- Traill ZC, Davies RJ, Gleeson FV. Thoracic Computed Tomography in Patients with Suspected Malignant Pleural Effusions. Clinical Radiology 2001;56:193-6. [Crossref] [PubMed]
- Yilmaz U, Polat G, Sahin N, et al. CT in differential diagnosis of benign and malignant pleural disease. Monaldi Arch Chest Dis 2005;63:17-22. [Crossref] [PubMed]
- Knuuttila A, Kivisaari L, Kivisaari A, et al. Evaluation of pleural disease using MR and CT: with special reference to malignant pleural mesothelioma. Acta Radiologica 2001;42:502-7. [PubMed]
- Duysinx B, Nguyen D, Louis R, et al. Evaluation of Pleural Disease With 18-Fluorodeoxyglucose Positron Emission Tomography Imaging. Chest 2004;125:489-93. [Crossref] [PubMed]
- Toaff JS, Metser U, Gottfried M, et al. Differentiation Between Malignant and Benign Pleural Effusion in Patients With Extra-Pleural Primary Malignancies. Invest Radiol 2005;40:204-9. [Crossref] [PubMed]
- Kwek BH, Aquino SL, Fischman AJ. Fluorodeoxyglucose Positron Emission Tomography and CT After Talc Pleurodesis. Chest 2004;125:2356-60. [Crossref] [PubMed]
- Heffner JE, Klein JS. Recent Advances in the Diagnosis and Management of Malignant Pleural Effusions. Mayo Clin Proc 2008;83:235-50. [Crossref] [PubMed]
- Lee YC, Baumann MH, Maskell NA, et al. Pleurodesis Practice for Malignant Pleural Effusions in Five English-Speaking Countries. Chest 2003;124:2229-38. [Crossref] [PubMed]
- Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg 2006;29:829-38. [Crossref] [PubMed]
- Detterbeck FC. Diagnosis and Treatment of Lung Cancer. W B Saunders Company; 2001:1.
- Cantó A, Ferrer G, Romagosa V, et al. Lung cancer and pleural effusion. Clinical significance and study of pleural metastatic locations. Chest 1985;87:649-52. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Decker DA, Dines DE, Payne WS, et al. The Significance of a Cytologically Negative Pleural Effusion in Bronchogenic Carcinoma. Chest 1978;74:640-2. [Crossref] [PubMed]
- Stefani A, Natali P, Casali C, et al. Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. Eur J Cardiothorac Surg 2006;30:827-32. [Crossref] [PubMed]
- Dziedzic D, Orlowski T. The Role of VATS in Lung Cancer Surgery: Current Status and Prospects for Development. Minim Invasive Surg 2015;2015:938430.
- Lee YT. Breast carcinoma: Pattern of metastasis at autopsy. J Surg Oncol 1983;23:175-80. [Crossref] [PubMed]
- Fentiman IS, Rubens RD, Hayward JL. Control of pleural effusions in patients with breast cancer a randomized trial. Cancer 1983;52:737-9. [Crossref] [PubMed]
- Banerjee AK, Willetts I, Robertson JF, et al. Pleural effusion in breast cancer: a review of the Nottingham experience. Eur J Surg Oncol 1994;20:33-6. [PubMed]
- Yi A, Kim HH, Shin HJ, et al. Radiation-Induced Complications after Breast Cancer Radiation Therapy: a Pictorial Review of Multimodality Imaging Findings. Korean J Radiol 2009;10:496. [Crossref] [PubMed]
- Singer TS, Sulkes A, Biran S. Pleural effusion in breast cancer: influence upon clinical course and survival. Chemioterapia 1986;5:66-9. [PubMed]
- Alexandrakis MG, Passam FH, Kyriakou DS, et al. Pleural Effusions in Hematologic Malignancies. Chest 2004;125:1546-55. [Crossref] [PubMed]
- Das DK, Gupta SK, Ayyagari S, et al. Pleural effusions in non-Hodgkin's lymphoma. A cytomorphologic, cytochemical and immunologic study. Acta Cytol 1987;31:119-24. [PubMed]
- Celikoglu F, Teirstein AS, Knellenstein DJ, et al. Pleural Effusion in Non-Hodgkin’s Lymphoma. Chest 1992;101:1357-60. [Crossref] [PubMed]
- Xaubet A, Diumenjo MC, Maŕin A, et al. Characteristics and prognostic value of pleural effusions in non-Hodgkin's lymphomas. Eur J Respir Dis 1985;66:135-40. [PubMed]
- Browse NL, Allen DR, Wilson NM. Management of chylothorax. Br J Surg 1997;84:1711-6. [Crossref] [PubMed]
- Romero S. Nontraumatic chylothorax. Curr Opin Pulm Med 2000;6:287-91. [Crossref] [PubMed]
- Graham DD, McGahren ED, Tribble CG, et al. Use of video-assisted thoracic surgery in the treatment of chylothorax. Ann Thorac Surg 1994;57:1507-11; discussion 1511-2. [Crossref] [PubMed]
- Inderbitzi RG, Krebs T, Stirneman T, et al. Treatment of postoperative chylothorax by fibrin glue application under thoracoscopic view with use of local anesthesia. J Thorac Cardiovasc Surg 1992;104:209-10. [PubMed]
- Kent RB, Pinson TW. Thoracoscopic ligation of the thoracic duct. Surg Endosc 1993;7:52-3. [Crossref] [PubMed]
- Stringel G, Teixeira JA. Thoracoscopic ligation of the thoracic duct. JSLS 2000;4:239-42. [PubMed]
- Murphy MC, Newman BM, Rodgers BM. Pleuroperitoneal shunts in the management of persistent chylothorax. Ann Thorac Surg 1989;48:195-200. [Crossref] [PubMed]
- Kintzer JS, Rosenow EC, Kyle RA. Thoracic and pulmonary abnormalities in multiple myeloma. A review of 958 cases. Arch Intern Med 1978;138:727-30. [Crossref] [PubMed]
- Kamble R, Wilson CS, Fassas A, et al. Malignant pleural effusion of multiple myeloma: prognostic factors and outcome. Leuk Lymphoma 2005;46:1137-42. [Crossref] [PubMed]
- Yokoyama T, Tanaka A, Kato S, et al. Multiple myeloma presenting initially with pleural effusion and a unique paraspinal tumor in the thorax. Intern Med 2008;47:1917-20. [Crossref] [PubMed]
- Bourantas KL, Tsiara S, Panteli A, et al. Pleural Effusion in Chronic Myelomonocytic Leukemia. Acta Haematologica 1998;99:34-7. [Crossref] [PubMed]
- Nuwal P, Dixit R, Dargar P, et al. Pleural effusion as the initial manifestation of chronic myeloid leukemia: Report of a case with clinical and cytologic correlation. J Cytol 2012;29:152-4. [Crossref] [PubMed]
- Linton A, Vardy J, Clarke S, et al. The ticking time-bomb of asbestos: its insidious role in the development of malignant mesothelioma. Critical Reviews in Oncology/Hematology. Elsevier 2012;84:200-12.
- Halstead JC, Lim E, Venkateswaran RM, et al. Improved survival with VATS pleurectomy-decortication in advanced malignant mesothelioma. Eur J Surg Oncol 2005;31:314-20. [Crossref] [PubMed]
- Valsecchi A, Arondi S, Marchetti G. Medical thoracoscopy: Analysis on diagnostic yield through 30 years of experience. Ann Thorac Med. Ann Thorac Med 2016;11:177-82. [Crossref] [PubMed]
- Katlic MR, Facktor MA. Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases. Ann Thorac Surg 2010;90:240-5. [Crossref] [PubMed]
- Metintas M, Ak G, Parspour S, et al. Local recurrence of tumor at sites of intervention in malignant pleural mesothelioma. Lung Cancer 2008;61:255-61. [Crossref] [PubMed]
- Cardillo G. Long-term follow-up of video-assisted talc pleurodesis in malignant recurrent pleural effusions. Eur J Cardiothorac Surg 2002;21:302-5; discussion 305-6. [Crossref] [PubMed]
- Schulze M, Boehle AS, Kurdow R, et al. Effective treatment of malignant pleural effusion by minimal invasive thoracic surgery: thoracoscopic talc pleurodesis and pleuroperitoneal shunts in 101 patients. Ann Thorac Surg 2001;71:1809-12. [Crossref] [PubMed]
- Rena O, Boldorini R, Papalia E, et al. Persistent lung expansion after pleural talc poudrage in non-surgically resected malignant pleural mesothelioma. Ann Thorac Surg 2015;99:1177-83. [Crossref] [PubMed]
- Ibrahim IM, Dokhan AL, El-Sessy AA, et al. Povidone-iodine pleurodesis versus talc pleurodesis in preventing recurrence of malignant pleural effusion. J Cardiothorac Surg 2015;10:64. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Available online: http://thorax.bmj.com/content/65/Suppl_2/ii32
- Waller DA. The role of surgery in diagnosis and treatment of malignant pleural mesothelioma. Curr Opin Oncol 2003;15:139-43. [Crossref] [PubMed]
- Shojaee S, Lee HJ. Thoracoscopy: medical versus surgical-in the management of pleural diseases. J Thorac Dis 2015;7:S339-51. [PubMed]
- Bueno R, Reblando J, Glickman J, et al. Pleural biopsy: a reliable method for determining the diagnosis but not subtype in mesothelioma. Ann Thorac Surg 2004;78:1774-6. [Crossref] [PubMed]
- Bhatnagar R, Laskawiec-Szkonter M, Piotrowska HEG, et al. Evaluating the efficacy of thoracoscopy and talc poudrage versus pleurodesis using talc slurry (TAPPS trial): protocol of an open-label randomised controlled trial. BMJ Open 2014;4:e007045. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Hunt BM, Farivar AS, Vallières E, et al. Thoracoscopic talc versus tunneled pleural catheters for palliation of malignant pleural effusions. Ann Thorac Surg 2012;94:1053-7; discussion 1057-9. [Crossref] [PubMed]
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and Safety of Tunneled Pleural Catheters in Adults with Malignant Pleural Effusions: A Systematic Review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Thomas R, Budgeon CA, Kuok YJ, et al. Catheter Tract Metastasis Associated With Indwelling Pleural Catheters. Chest 2014;146:557-62. [Crossref] [PubMed]
- Bertolaccini L, Viti A, Terzi A. To seed or not to seed: the open question of mesothelioma intervention tract metastases. Chest 2014;146:e111. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Mahidhara RS. A propensity-matched comparison of pleurodesis or tunneled pleural catheter in patients undergoing diagnostic thoracoscopy for malignancy. Ann Thorac Surg 2013;96:259-63; discussion263-4.
- Davies H, Mishra EK, Wrightson JM, et al. The Second Therapeutic Intervention In Malignant Effusion Trial (TIME2): A Randomised Controlled Trial To Assess The Efficacy And Safety Of Patient Controlled Malignant Pleural Effusion Drainage By Indwelling Pleural Catheter Compared To Chest Tube And Talc Slurry Pleurodesis. American Thoracic Society; 2012:A6861-1.
- Mishra E, Davies H, Wrightson J, et al. The second therapeutic intervention in malignant effusion trial (TIME2): A randomised controlled trial to assess the efficacy and safety of patient controlled malignant pleural effusion drainage by indwelling pleural catheter compared to chest drain and talc slurry pleurodesis. Eur Respir J 2014.40.
- Martin-Ucar AE. Palliative surgical debulking in malignant mesothelioma Predictors of survival and symptom control. Eur J Cardiothorac Surg 2001;20:1117-21. [Crossref] [PubMed]
- Waller DA, Morritt GN, Forty J. Video-Assisted Thoracoscopic Pleurectomy in the Management of Malignant Pleural Effusion. Chest 1995;107:1454-6. [Crossref] [PubMed]
- Grossebner MW, Arifi AA, Goddard M, et al. Mesothelioma--VATS biopsy and lung mobilization improves diagnosis and palliation. Eur J Cardiothorac Surg 1999;16:619-23. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Rintoul RC, Tod A, Sivasothy P, et al. A feasibility study of indwelling pleural catheter versus VAT pleurecotomy for trapped lung in mesothelioma. (Page 134). Available online: http://imig2016.org/wp-content/uploads/2016/04/iMig-2016-Abstract-Book.pdf
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Determinants of Survival in Malignant Pleural Mesothelioma: A Surveillance, Epidemiology, and End Results (SEER) Study of 14,228 Patients. Sung SY, editor. PLOS ONE. Public Library of Science; 2015;10:e0145039.
- Mineo TC. Malignant Pleural Mesothelioma: Present Status and Future Directions. Bentham Science Publishers; 2016:1.
- Edwards JG, Abrams KR, Leverment JN, et al. Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax 2000;55:731-5. [Crossref] [PubMed]
- Francart J, Vaes E, Henrard S, et al. A prognostic index for progression-free survival in malignant mesothelioma with application to the design of phase II trials: A combined analysis of 10 EORTC trials. Eur J Cancer 2009;45:2304-11. [Crossref] [PubMed]
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma from the International Mesothelioma Interest Group. Lung Cancer 1996;14:1-12. [Crossref] [PubMed]
- Nowak AK, Chansky K, Rice DC, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2089-99.
- Rice D, Chansky K, Nowak A, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol; 2016;11:2100-11.
- Rusch VW, Chansky K, Kindler HL, et al. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol 2016;11:2112-9. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Stahel RA, Riesterer O, Xyrafas A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol 2015;16:1651-8. [Crossref] [PubMed]
- Opitz I. Management of malignant pleural mesothelioma-The European experience. J Thorac Dis 2014;6 Suppl 2:S238-52. [PubMed]
- Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4; author reply 1094-5. [Crossref] [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, 2012, Boston: Mass; 2013; 909-10.
- Baud M, Bobbio A, Lococo F, et al. Should we continue to offer extrapleural pneumonectomy to selected mesothelioma patients? A single center experience comparing surgical and non-surgical management. Jpn J Clin Oncol 2014;44:1127-9. [Crossref] [PubMed]
- Edwards JG, Stewart DJ, Martin-Ucar A, et al. The pattern of lymph node involvement influences outcome after extrapleural pneumonectomy for malignant mesothelioma. J Thorac Cardiovasc Surg 2006;131:981-7. [Crossref] [PubMed]
- Nakas A, Waller D, Lau K, et al. The new case for cervical mediastinoscopy in selection for radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2012;42:72-6; discussion76.
- Waller DA. The staging of malignant pleural mesothelioma: are we any nearer to squaring the circle? Eur J Cardiothorac Surg 2016;49:1648-9. [Crossref] [PubMed]
- Martin-Ucar AE, Stewart DJ, West KJ, et al. A median sternotomy approach to right extrapleural pneumonectomy for mesothelioma. Ann Thorac Surg 2005;80:1143-5. [Crossref] [PubMed]
- Demmy TL. Video-assisted thoracoscopic extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:533. [PubMed]
- Stewart DJ, Martin-Ucar AE, Edwards JG, et al. Extra-pleural pneumonectomy for malignant pleural mesothelioma: the risks of induction chemotherapy, right-sided procedures and prolonged operations. Eur J Cardiothorac Surg 2005;27:373-8. [Crossref] [PubMed]
- Waller DA. Lung-sparing total pleurectomy: the surgical option of choice in malignant pleural mesothelioma? Eur J Cardiothorac Surg 2013;44:123-4. [Crossref] [PubMed]
- Sharkey AJ, Tenconi S, Nakas A, et al. The effects of an intentional transition from extrapleural pneumonectomy to extended pleurectomy/decortication. Eur J Cardiothorac Surg 2016;49:1632-41. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Lim E. Surgery for Mesothelioma —MARS 2 update 2017. Available online: https://www.drericlim.com/news/2017/1/1/surgery-for-mesothelioma-mars-2-update-2017
- Sharkey AJ. Is there any role for video assisted partial pleurectomy after MesoVATS? 2014.
- Nakas A, Tsitsias T, Waller D. O-072 * is there any benefit in lung sparing macroscopic complete resection over video-assisted debulking in malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 2013;17:S19-9. [Crossref]
- Rice D, Rusch V, Pass H, et al. Recommendations for Uniform Definitions of Surgical Techniques for Malignant Pleural Mesothelioma: A Consensus Report of the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group. J Thorac Oncol 2011;6:1304-12. [Crossref] [PubMed]
Cite this article as: Perikleous P, Waller DA. Video assisted thoracoscopic and open chest surgery in diagnosis and treatment of malignant pleural diseases. J Vis Surg 2017;3:85.