Materials and techniques in chest wall reconstruction: a review
Introduction
Chest wall integrity and stability are the main factors that ensure the protection of intrathoracic organs and an adequate respiratory function. The thoracic surgeon often has to deal with neoplastic, traumatic and malformative diseases affecting the chest wall and requiring the demolition and reconstruction or stabilization of the thoracic cage. For this purpose many techniques have been proposed including the use of various materials, but to date there are still no clear guidelines in the management of chest wall diseases (1).
The first chest wall reconstruction was described by Tensini in 1906 when a pedicled latissimus dorsi flap was used to cover an anterior chest wall defect (2). Since that time, chest wall reconstruction has evolved significantly as surgical techniques have advanced and multiple prosthetic and bioprosthetic materials have become available (3). Chest wall defects generally result from resection of primary chest wall tumours, locally-invasive malignancies or metastatic lesions (4). The optimal approach to reconstruction is determined by the size, location and depth of the defect, viability of the surrounding tissue and prior operative procedures (5), because in case of neoplastic pathology it’s mandatory to obtain oncologic margins not compromised and this could be result in large full-thickness defects (6), often resulting in a significant morbidity and mortality for the patient (3,7-10).
Most surgeons agree that defects >5 cm in diameter or including >4 ribs should be reconstructed due to high risk of lung herniation and respiratory compromise from paradoxical motion of the chest wall, particularly true for anterolateral defects and full thickness resections (3,5,11). In contrast, some apicoposterior defects, even 10 cm in size, do not require reconstruction because of the support provide by scapula and shoulder girdle, with the exception of defects lower than 4th rib posteriorly, with the tip of the scapula at risk to entrapment (3,12,13). With the increased availability of reconstruction materials, then, and particularly biologic materials, some surgeons proposed the reconstruction of nearly all chest wall defects, with the objective to avoid patient perception of chest wall instability (3,5,13).
The primary goals of all chest wall reconstructions are to obliterate dead space, restore chest wall rigidity, preserve pulmonary mechanic, protect intrathoracic organs, provide soft tissue coverage, minimize deformity and allow patients to receive adjuvant radiotherapy if indicated (3,9). Therefore, a multidisciplinary approach, including input from thoracic surgeons, plastic surgeons, neurosurgeons as well as medical and radiation oncologists is essential.
Actually several synthetic, biologic, and metallic materials are available to reconstruct the chest wall defects, but each prosthetic material has its own advantages and disadvantages and none have proven to be clearly superior (4,12). In particular, the benefit of each material and technique of reconstruction need to be weighed against to main indicators, as the risk of infection and other major complications that could be fail the reconstructive result.
The recent advance in allograft and homograft production have provided new alternatives for restoring structural stability, preventing the infective complications (3,14).
We would describe the main reconstructive techniques and materials more adopted on Literature reports and an overview to the incoming future of chest wall reconstruction.
Synthetic, biologic and titanium meshes
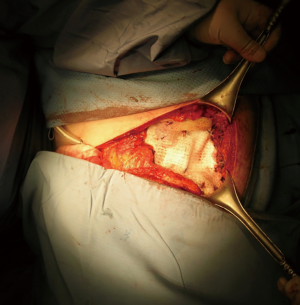
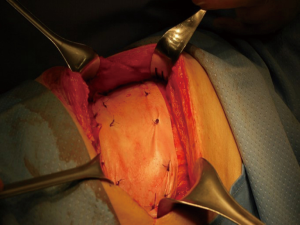
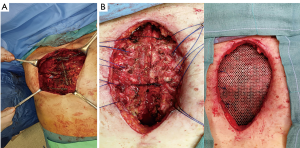
The use of a metal prostheses was first reported by a French surgeon in 1909 (15), but in the 1940s better-tolerated and easier to use materials, as plastic components emerged, modifying the modern era of chest wall reconstruction (16). Actually, a magnitude of synthetic meshes exist and present the advantages of easy manipulation and handling. They, more or less, all comply with the characteristics of ideal prosthetic material as determined by Le Roux and Sherma (17): (I) rigidity to abolish paradoxical movement; (II) inertness to allow in-growth of fibrous tissue and decrease the likelihood of infection; (III) malleability to fashion to the appropriate shape at the time of operation; and (IV) radiolucency to create an anatomic reference to do a better follow up and identify a possible local neoplastic relapse. Most patches are non-absorbable synthetic woven meshes: polypropylene, polyester and polytetrafluoroethylene (PTFE) soft tissue patches, usually doubled over and sutured to adjacent ribs and fascia to cover the immediate surface of the chest wall defect (Figure 1). These materials can be stretched uniformly in all directions, allowing uniform tension strength at the bone defect edges. They are simple to use and usually well tolerated when completely covered by viable tissue, provide a barrier that prevents fluid and air moving between pleural and subcutaneous space and propose a scaffold for the in-growth of regenerative connective tissue colonizing their outer and inner surfaces. Some authors reported an infection rate between 10% and 25% for synthetic meshes and the needs to remove the infected mesh to resolve the problem (8,16). In these cases a Vicryl mesh could be considered due to its inert, nonantigenic, biocompatible and slowly absorbing material or a biologic mesh as like as bovine pericardium prosthesis (Figure 2), that have the same tensile strength and elasticity as those synthetic, but some proper physiologic properties of resistance to infection and contamination. Consisting of acellular organic collagen-based matrices it allows for native tissue re-growth and revascularization, stimulating regeneration as opposed to scarring with minimal inflammatory response and less inclination to rejection. Differently to synthetic meshes, it can be placed directly over the lung and viscera without any complications, but not resulting in a rigid reconstruction of the thoracic wall, even if the stability achieved is enough to prevent paradoxical motions or respiratory distress (18,19). The only limitation is the elevated costs (16).
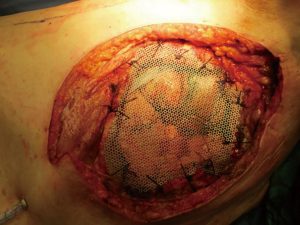
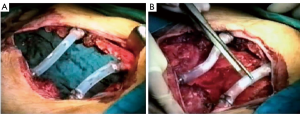
Recently there were proposed titanium meshes (MDF Medica) that present more strength than synthetic meshes, maintaining the same plasticity and adaptability on the chest wall defect. The 5-mm thickness mesh could obtain the right rigidity on the chest wall, preventing endothoracic organs lesions, preserving the system by infection for the inert condition of titanium and resulting well tolerated by the patient (Figure 3).
All these meshes often result safe and useful to resolve the thoracic wall defect, but for anterior, sternal or lower posterior defects could be insufficient, even if well sutured and stretched as a drum, to protect the inner organs. This problem should be resolved with the use of composite implant techniques (3,5,16).
Methyl methacrylate
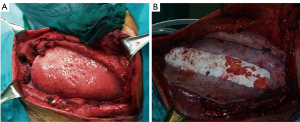
Methyl Methacrylate is usually sandwiched between two layers of the mesh to strengthen the rigidity of the reconstruction. Since 1980s and for many years this product has been the best choice to reconstruct the sternum, ribs and chest wall, entirely or partially (18). It could be prepared on the surgical field, where a first layer of polypropylene mesh is fixed straight on the base of the rib defect and the methyl methacrylate substitute is then added to the defect shape and covered with a second layer of the prosthesis, hardening an exothermic reaction and becoming rigid and forming a cast that conforms to the defect; or, most commonly, the prosthetic system is designed in the same manner on the back table (16,20,21)). With this technique, most of all for extensive anterior and lateral chest wall defects, paradoxical movements and chest deformities are prevented (16,20,21) (Figure 4).
However methylmethacrylate material seems not to be permeable to fluids and therefore are considered to increase pain (3,4) and excessive chest wall rigidity (4). A fracture of the methacrylate (22) is possible and most of all increases the risk of infection (23). Wound complications are reported on 10–20% of patients at 90 days, requiring the removal of the prostheses in approximately 5% of patients (4,22). Nevertheless several case series reported on important functional results, without an high infection rate (4,5,16). Most of the authors agree that complete coverage with viable soft tissue in this chest wall reconstruction is an essential step to minimize the risk of local complications (4,5,16,23).
Other composite implant techniques, applying silicone tubes, rubber and Carbone fiber systems have been described in case reports (11,23,24,25).
PTFE
PTFE (GORE-TEX) is another material well-suited and commonly used for chest wall reconstruction. Similar to methyl methacrylate, PTFE is watertight and causes minimal foreign body reaction; however, it is flexible, allowing it to conform to the chest wall. Most commonly 2 mm thick PTFE mesh is stretched over the chest wall defect using heavy permanent suture. To provide chest wall stability, it is important to pull the mesh as tight as possible, with sutures laced around or through adjacent ribs (3). A bone drill or sharp towel clip works well, creating holes in ribs for fixation. PTFE can be used to stabilize large chest wall defects and should be completely covered with viable tissue after implantation. Its use is absolutely contraindicated in infected fields. However, if the mesh became infected and the patient is not septic, immediate removal is not always indicated. Seder et al. (3) propose to remove it after 6–8 weeks later, when enough scar tissue often forms, to support the chest wall after the infected mesh is removed.
Titanium plates
About 5 years ago, considering the favourable experiences obtained with titanium implants in other fields of prosthetic surgery (orthopedic, maxillo-facial surgery) a new dedicated titanium plates system was introduced for the treatment of the chest wall diseases (1,26,27). This system was introduced to support the chest wall reconstruction after demolition for neoplastic disease, as like as to fix the fractures of the thoracic cage after trauma and sternal dehiscence. Titanium is an ideal prosthetic material, as it has an high resistance to corrosion, a low specific weight, a remarkable resistance to traction, it is diamagnetic and compatible with MRI, but, above all, is biologically inert and highly biocompatible.
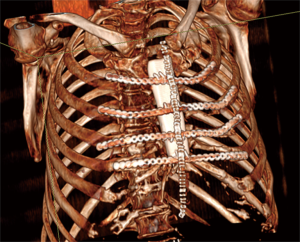
Actually there are several types of rib prosthesis systems, the oldest one is the Borrelly steel staple-splint system, very popular in the 1990s (26). The STRATOS system could be an evolution of the previous system, securing to the rib ends clips that resemble claws at the two ends of the bar. The MatrixRIB and MDF Medica devices use a comfortable well-remodellable bar with holes for screws to threated the bar to the ribs or the sternum. To obtain an optimal fixation, it is fundamental to lock screws to the bone bicortically and to use at least three screws for blocking each side of the bar. It’s adaptable to a wide variety of chest wall defects, allowing to recreate the anatomical and physiological appearance of the thoracic cage (Figure 5). Some reports in the literature confirmed the easy use and the rapid learning curve of this device, that is very useful for clavicle substitution too (Figure 6) (1,27,28). However, we must consider that in trauma patients titanium plates are always the only material used for chest wall stabilization, while in neoplastic cases the reconstruction of the chest wall requires integration with traditional techniques, such as synthetic biologic or titanium meshes (Figures 7,8) and various muscle flaps. Many authors (1,25-28) agree that titanium system represents a better solution in the reconstruction of large full-thickness defects, restoring the rigidity of the thoracic cage and preventing respiratory and infective complications. Few complications, as plate fracture, bar dislocation and thoracic pain, are described for this system. Bar fracture rate varied from 0 to 11% in some series; plate dislocation frequently is due to the mismatch between the screws length and rib thickness, or the destruction of the bone threads that lock the screws into the rib, due to repeating re-drilling in the same hole (23).
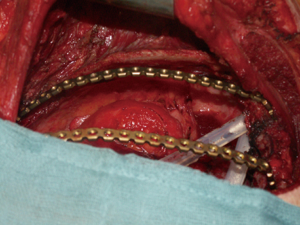
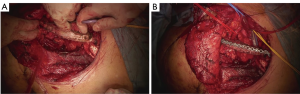
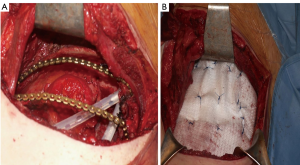
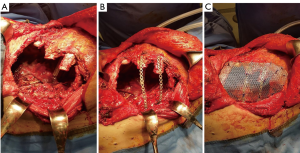
Particularly, this system is very safe in sternal traumas and tumours. The sternum is very important for chest wall stability and its integrity is mandatory for respiratory mechanism. Titanium plates and meshes permit to restore the sternal defect reconstructing its integrity in traumas (29) and recreating an anterior stability of the thoracic cage in partial or total sternectomies (Figures 9,10) (30,31). Regarding the sternal reconstruction, other systems are described in some series. Dahan et al. (32) described an easy technique to reconstruct the sternal floor applying Kirschner’s wires in the spongiest aspect of the cut ribs, associated with silicone molds that are threated on ribs and wires and tied with ligatures at both the extremities. Methylmethacrylate is injected in the mold to fill it totally (Figure 11). This technique provide excellent stability and offer a suitable support to receive a regional or omental flap (16,32).
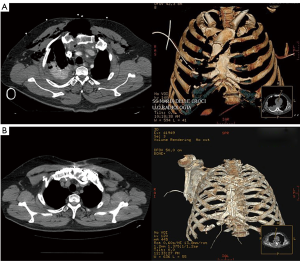
Other dedicated sternal prostheses are the Ley prostheses, a very thick titanium alloy plate shaped as a stepladder. This device, initially designed for stabilization of the sternum after post-operative mediastinitis, results flexible and adapting to sternal silhouette. Pedersen reported the application of Ley prostheses in the reconstruction of 3 cases of sternal chondrosarcoma with good results (33).
Watanabe et al. reported on the use of a sternal ceramic prosthesis constituted of hydroxyapatite and tricalcium phosphate (Ceratite) creating a customized prosthetic bone tailored to the anterior thoracic wall defects with slots and holes in the Ceratite prosthesis as fasteners. It’s an original idea with many advantages, as the possibility to provide the useful template for bone growth, strength and biocompatibility but the great disadvantage of excessive cost (24).
Allograft and homograft
Both human and porcine bioprosthetic materials have been developed over the past decade in response to the need for complex chest wall reconstruction in infected irradiated and re-operative fields. Cryopreserved allografts and homografts, recovered from cadaveric donors and stored at –80°, are being more commonly used for restore structural integrity in large chest wall defects (3,22). These materials represent a potentially limitless source for chest wall reconstruction and have been shown to exhibit differences in cytotoxicity, bacterial adhesion and biomechanical properties, compared to traditional prosthetics (34). The major advantage is that they are able to incorporate into native tissue with revascularization and cellular repopulation, making them more resistant to infection and useful in contaminated fields (22,34).
Sternal allograft transplantation represents an ideal example of allograft for anterior chest wall reconstruction following sternectomy for tumors or infective processes. Bone grafts act as a structure for osteoprogenitor cells and bone growth. These materials are already extensively used in orthopedic and maxillofacial surgery with very good results (35-38). The main limitation of bone autografts concerns the amount of bone that can be harvested and thus implanted in the thoracic site of reconstruction.
In the last years, a large numbers of case reports described the use of bone allografts also for chest wall reconstruction (14,35,39-41) (Figure 12). In 2010, the Padua Group reported the first case of allograft sternochondral replacement after sternectomy for chondrosarcoma (14).
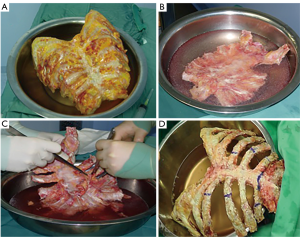
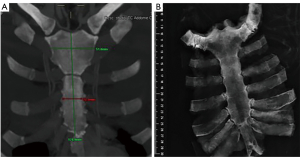
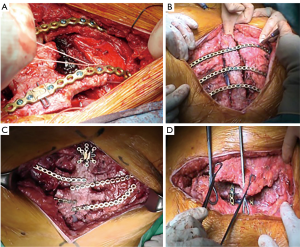
The graft, conserved in tissue bank, undergoes a 72-hours washing in sterile saline solution added with antibiotics and then it is irradiated and stored at –80 °C. The day before the surgical procedure, the graft is defrosted at 4–6 °C for 12 hours and is immersed in sterile NaCl 0.9% solution added with antibiotics and collected at 4–6 °C until used (42,43) (Figure 13). The reconstruction of the anterior chest wall after partial or complete sternectomy using a sternal allograft is simple, using the preoperative CT scan of the recipient and an allograft radiograph to measure the longitudinal and transverse diameters of the patient and allograft sternum at the level of the sternal clavicular junction, manubrium and the sternal body, to guarantee the correct matching between the donor and recipient sterni (Figure 14) (14,43). Intraoperatively, the presence of any discrepancy between the allograft and the surgical site of implantation can be easily corrected by, tailoring the bone allograft with a saw and rasp. Recently, new technologies such as 3D-printers and computed based navigation surgery appear to be very promising in preoperative planning and simplification in matching the allograft bone and the recipient’s chest wall (44,45). At the end of the operation, a quick, safe and efficient stabilization of the transplanted bone is achieved with titanium plates and screws, usually placed between the allograft and the ribs. Whenever possible, a pectoralis muscle flap must cover the allograft, to further reduce the infective risk and to prevent the cutaneous decubitus of titanium bars (42) (Figure 15).
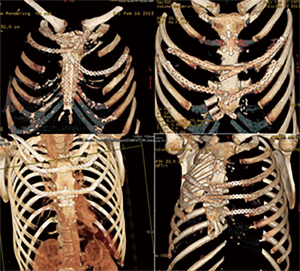
Stella et al. (42) published their series regarding sternal allograft transplantation, describing 4 cases with no mortality or morbidity related to the surgical technique even after a mean follow-up of 9.7 months (Figure 16) and a recent multicenter study, regarding 18 patients underwent allograft sternal transplantation, reported excellent long-term results, with a mean follow-up time of 36 months. In this series, none of the patients had complications related to the implanted allograft, with a good chest wall stability and optimal respiratory function (46,47).
Soft tissue coverage
Regardless of the technique used to establish skeletal stability, full tissue coverage of the prosthesis is mandatory, using direct suture, skin grafts, local advancement flaps, pedicled myocutaneous flaps or free flaps. In this case, the plastic surgeon often plays a leading role in extensive chest wall defects reconstruction. Luckily, the thorax has a wide array of large muscle groups, that can be use individually or in combination. The selection of the appropriate tissue transfer necessitates evaluation of surgical scars, dimension of the defect, need for prosthetic material or viscera coverage, and whether additional tissue bulk is required to fill a dead space void (3).
Among the several muscles used, the latissimus dorsi is considered the workhorse in chest wall reconstruction, as this flap enables coverage of the entire ipsilateral chest wall (1,3,5) (Figure 17). It is based of the thoracodorsal artery from subscapular artery, which originates at the axillary artery (48), but it can survive off of retrograde flow from the serratus branch into the thoracodorsal artery (49). The choice of blood supply is determined by the arc of rotation required to cover the given defect. The latissimus can provide a flap up to 105 cm2 in females and 195 cm2 for males (49), and could be used to cover anterolateral and posterior wall defects or can be passed, after resection of a portion of 2nd or 3rd rib to avoid vascular compression, between the ribs to fill a significant amount of intrathoracic space (3). The large diameter of the vascular pedicle allows the latissimus dorsi to be used as a free graft (1,3,5,16). Persistent seroma is the most common complication of this technique, described in up to 79% of cases (50). In addition it is possible a temporary functional disability with poor arm abduction between 90° and 180°, with reduction in arm strength, commonly resolved in the first year (51).
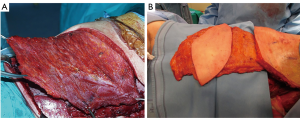
The pectoralis major is taken into account as the principal flap for sternal and anterosuperior chest wall defect coverage (52,53). Its blood supply is duplex: the dominant comes from the thoracoacromial pedicle, the other from sixth intercostal perforating branches arising from mammary artery (5). This dual vascular pattern permits the application of only the medial 2/3 of the muscle, sparing the lateral third, or a complete mobilization of the muscle, as an island flap based on dominant pedicle, extending the reach of this flap to the xiphoid process, obliterating the entire anterior mediastinal space (5). If bilateral pectoralis flaps are mobilized to cover the sternum and a future sternal resection is required, the flaps can be usually be preserved and re-applied to the sternum (Figure 18). This muscle can be, as latissimus dorsi, passed through an intercostal space to fill an apical intrathoracic dead space (3,5,20).
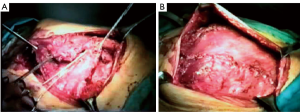
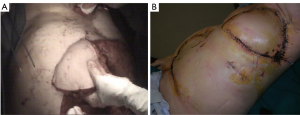
The rectus abdominis flap, feeded by deep superior or inferior epigastric systems, affords unparalleled versatility of skin island design [vertically (VRAM) – transversed (TRAM) oriented skin pedicle], which has enabled wide application of this flap for coverage of anterior or anterolateral defects. VRAM skin islands have a more robust blood supply than TRAM islands due to the increased number of perforators. (5) VRAM flaps are well-suited for covering large longitudinal chest wall defects, such as after total sternectomy. TRAM flaps can cover defects up to 40 cm in size and are most often proposed to supply soft tissue coverage of the anterolateral thoracic cage (Figure 19). Abdominal hernias after TRAM and VRAM are the most significant morbidity, with an incidence rate of about 13% of the cases (49,54).
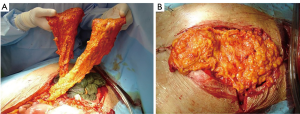
Free muscle flaps may be indicated if local muscle group have been resected, previously injured or irradiated (3). This technique can play an important role in chest wall reconstruction and was possible with the evolution of microsurgical techniques and allow a 2-team approach to minimize operative time. On the back (latissimus dorsi, parascapular) a fasciocutaneous or myocutaneous flaps, on the thigh, tensor fasciae latae and TRAM are some of free flaps usually used. The internal mammary artery represents the main connecting vessel at anterior chest wall, while thoracodorsal vessels do the same action for lateral site. If there is a lack of vascular feeding an arteriovenous loop between the cephalic vein and the thoracoacromial artery can be an effort-intensive but safe option (3). Donor-site morbidity of free flap harvest is relatively low, particularly if the donor site is repaired primarily (55). The postoperative care of free flaps requires frequent inspections, strict positioning protocols, and may require anticoagulation (3).
The omentum majus flap (Figure 20) is another great option for repairing defects in the anterior chest wall, in case of the aforementioned pedicled flaps or free flaps fail or are not suitable. Pedicled to the unilateral or bilateral gastro-omental vessels, it can be lifted virtually to any chest wall location via a laparotomic incision or, most of the cases, a laparoscopic procedure (56). The size of omentum flap can be determined under direct visual control during surgical procedure. The omentum majus is pulled up from the stomach to achieve the correct rotatory radius and is transposed to the thoracic cage. This flap should be raised only by experienced surgeons, able to treat the potential intra-abdominal complications (i.e., intestinal perforations or bleeding). The great plasticity of the omentum permits to use it for sealing dead space, but it must always be covered by skin graft and it’s possible a partial secondary healing, due to persistent seroma from fatty tissue necrosis. Although its versatility it presents significant potential intra-abdominal morbidity and so remains mainly a fallback option when other procedures fail or are not fitting (5,56).
A look into the future
Despite the recent advances in titanium prosthetic bar design, actuated to repair many chest wall defect configurations, the prosthesis are far from ideal yet (22). There were good results using bioabsorbable materials for chest wall repair, mainly important in the growing pediatric patients (30,57). A new recent development could be a computed tomography with reconstructed 3-dimensional (3D) images, that could guide the production, via a 3D printing technology, accurate resin, polymer, metal and degradable biomaterial prosthesis. A combination of materials can be used, some biodegradable, others to make rigid the structure and more exciting evolution should be a 3-D printing bioscaffold, that allows the growth and colonization by patient’s own cells into (58,59).
These evolutions tend to have a prosthesis fitted on patient’s habitus and disease. Metcalfe and Ferguson suggested that all skin layers could be replaced using a combination of biomaterials, wound healing, embryonic development stem cell and regeneration (60). Biodegradable materials, like collagen-coated polydioxanone and polycaprolactone, have been recently investigated. A polydioxanone mesh demineralized bone and bone marrow stromal cells has been successfully used in an animal model to replace ribs and reconstruct a relatively small chest wall defect (61).
In conclusion, the reconstruction of extensive chest wall defects following thoracic wall resection, could be a formidable challenge. This can be achieved by adhering to the principles of biomimesis, in which anatomy is respected, function is preserved, optimal reconstructive materials are chosen and a multidisciplinary approach to complex reconstruction is undertaken. After an R0 chest wall resection, first skeletal stability must be established with prosthetic or bioprosthetic materials, or a combination of both. It is imperative that soft tissue coverage be achieved, using one of multiple available rotational, advancement or free flaps. This procedure requires a precise understanding of neurovascular anatomy of muscle group, to ensuring a successful soft tissue transfer. With the new rapid evolutions in biodegradable scaffoldings and innovation in surgical techniques, outcomes for extensive chest wall reconstruction are expected to continue to improve.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- De Palma A, Sollitto F, Loizzi D, et al. Chest wall stabilization and reconstruction: short and long-term results 5 years after the introduction of a new titanium plates system. J Thorac Dis 2016;8:490-8. [Crossref] [PubMed]
- Tensini I. Sopra il mio nuovo processo di amputazione della mammella. Gazzetta Med Ital 1906;57:141-2.
- Seder CW, Rocco G. Chest wall reconstruction after extended resection. J Thorac Dis 2016;8:S863-S871. [Crossref] [PubMed]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Ferraro P, Cugno S, Liberman M, et al. Principles of chest wall resection and reconstruction. Thorac Surg Clin 2010;20:465-73. [Crossref] [PubMed]
- McAfee MK, Pairolero PC, Bergstralh EJ, et al. Chondrosarcoma of the chest wall: factors affecting survival. Ann Thorac Surg 1985;40:535-41. [Crossref] [PubMed]
- Geissen NM, Medairos R, Davila E, et al. Number of ribs resected is associated with respiratory complications following lobectomy with en bloc chest wall resection. Lung 2016;194:619-24. [Crossref] [PubMed]
- Daigeler A, Druecke D, Hakimi M, et al. Reconstruction of the thoracic wall long-term follow-up including pulmonary function tests. Langenbecks Arch Surg 2009;394:705-15. [Crossref] [PubMed]
- Tukiainen E, Popov P, Asko-Seljavaara S. Microvascular reconstructions of full-thickness oncological chest wall defects. Ann Surg 2003;238:794-801. [Crossref] [PubMed]
- van Geel AN, Wouters MW, Lans TE, et al. Chest wall resection for adult soft tissue sarcomas and chondrosarcomas. Analysis of prognostic factors. World J Surg 2011;35:63-9. [Crossref] [PubMed]
- Mahabir RC, Butler CE. Stabilization of the chest wall: autologous and alloplastic reconstructions. Semin Plast Surg 2011;25:34-42. [Crossref] [PubMed]
- Deschamps C, Tirnaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall recontruction. J Thorac Cardiovasc Surg 1999;117:588-91. [Crossref] [PubMed]
- Rocco G. Chest wall resection and reconstruction according to the principles of biomimesis. Semin Thorac Cardiovasc Surg 2011;23:307-13. [Crossref] [PubMed]
- Marulli G, Harnad AM, Cogliati E, et al. Allograft sternochondral replacement after resection of large sternal chondrosarcoma. J Thorac Cardiovasc Surg 2010;139:e69-70. [Crossref] [PubMed]
- Gangolphe L. Enorme enchondrome de la fourchette sternale. Lyon Chir 1909;2:112.
- Thomas PA, Brouchet L. Prosthetic reconstruction of the chest wall. Thorac Surg Clin 2010;20:551-8. [Crossref] [PubMed]
- le Roux BT, Sherma DM. Resection of tumors of the chest wall. Curr Probl Surg 1983;20:345-86. [Crossref] [PubMed]
- Butler CE, Langstein HN, Kronowitz SJ. Pelvic abdominal and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg 2005;116:1263-75. [Crossref] [PubMed]
- Holton LH, Chung T, Silverman RP, et al. Comparison of acellular dermal matrix and synthetic mesh for lateral chest wall reconstruction in a rabbit model. Plast Reconstr Surg 2007;119:1238-46. [Crossref] [PubMed]
- McCormack P, Bains MS, Beattle EJ Jr, et al. New trends in skeletal reconstruction after resection of chest wall tumors. Ann Thorac Surg 1981;31:45-52. [Crossref] [PubMed]
- Lardinois D, Muller M, Furrer M, et al. Functional assessment of chest wall integrity after metylmethacrylate reconstruction. Ann Thorac Surg 2000;69:919-23. [Crossref] [PubMed]
- Ng CS. Recent and future developments in chest wall reconstruction. Semin Thorac Cardiovasc Surg 2015;27:234-9. [Crossref] [PubMed]
- Chapelier AR, Missana MC, Couturaud B, et al. Sternal resection and reconstruction for primary malignant tumors. Ann Thorac Surg 2004;77:1001-6. [Crossref] [PubMed]
- Watanabe A, Watanabe T, Obama T, et al. new material for reconstruction of the anterior chest wall including the sternum. J Thorac Cardiovasc Surg 2003;126:1212-4. [Crossref] [PubMed]
- Harati K, Kolbenschlag J, Behr B, et al. Thoracic wall reconstruction after tumor resection. Front Oncol 2015;5:247-57. [Crossref] [PubMed]
- Borrelly J, Grosdidier G, Boileau S, et al. Chirurgie plastique de la paroi thoracique (deformations et tumeurs) a l’aide de l’attelle.agrafe à glissieres. Ann Chir Plast Esthet 1990;35:57-61. [PubMed]
- Iarussi T, Pardolesi A, Camplese P, et al. Composite chest wall reconstruction using titanium plates and mesh preserves chest wall function. J Thorac Cardiovasc Surg 2010;140:476-7. [Crossref] [PubMed]
- Billè A, Okiror L, Karenovics W, et al. Experience with titanium devices for rib fixation and coverage of chest wall defects. Interact Cardiovasc Thorac Surg 2012;15:588-95. [Crossref] [PubMed]
- Harston A, Roberts C. Fixation of sternal fractures: a systematic review. J Trauma 2011;71:1875-9. [Crossref] [PubMed]
- Fabre D, El Batti S, Singhal S, et al. a paradigm shift for sternal reconstruction using novel titanium rib bridge system following oncological resections. Eur J Cardiothorac Surg 2012;42:965-70. [Crossref] [PubMed]
- Chapelier A. Resection and reconstructionfor primary sternal tumors. Thorac Surg Clin 2010;20:529-34. [Crossref] [PubMed]
- Dahan M, Brouchet L, Berjaud J, et al. Chirurgie des tumeurs d la paroi thoracicque. Ann Chir Plast Esthet 2003;48:93-8. [Crossref] [PubMed]
- Pedersen TAL, Pilegaard HK. Reconstruction of the thorax with Ley Prosthesis after resection of the sternum. Ann Thorac Surg 2009;87:e31-3. [Crossref] [PubMed]
- Wiegmann B, Korossis S, Burgwitz K, et al. In vistor comparison of biological and synthetic materials for skeletal chest wall reconstruction. Ann Thorac Surg 2015;99:991-8. [Crossref] [PubMed]
- Kaláb M, Karkoska J, Kaminek M, et al. Reconstruction of massive post-sternotomy defects with allogenic bone graft: four year results and experience using the method. Interact Cardiovasc Thorac Surg 2016;22:305-13. [Crossref] [PubMed]
- Heller L, Huang WC, Cheng HC, et al. Vascularized iliac bone flap used for sternum reconstruction after resection of chondrosarcoma. Plast Reconstr Surg 2002;110:1088-91. [Crossref] [PubMed]
- Chai Y, Zhang G, Shen G. Autogenous rib grafts for reconstruction of sternal defects after partial resection: a new surgical technique. Plast Reconstr Surg 2008;121:353e-355e. [Crossref] [PubMed]
- Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001;10:S96-101. [Crossref] [PubMed]
- Granero-Molto F, Weis JA, Longobardi L, et al. Role of mesenchimal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther 2008;8:255-68. [Crossref] [PubMed]
- Dell'amore A, Nizar A, Dolci G, et al. Sternal resection and reconstruction for local recurrence of breast cancer using the sternal alograft transplantation technique. Heart Lung Circ 2013;22:234-8. [Crossref] [PubMed]
- Dell'Amore A, Dolci G, Cassanelli N, et al. A massive post-sternotomy sternal defect treated by allograft sternal transplantation. J Card Surg 2012;27:557-9. [Crossref] [PubMed]
- Stella F, Dell'Amore A, Dolci G, et al. Allogenic sternal transplant after sternectomy for metastasis of ovarian carcinoma. Ann Thorac Surg 2012;93:e71-2. [Crossref] [PubMed]
- European association of tissue banks. General standards for tissue Banking. Vienna: OBIG-Transplant, 1995.
- Dell'Amore A, Cassanelli N, Dolci G, et al. An alternative technique for anterior chest wall reconstruction: the sternal allograft transplantation. Interact Cardiovasc Thorac Surg 2012;15:944-7. [Crossref] [PubMed]
- Ng CS. Recent and future developments in chest wall reconstruction. Semin Thorac Cardiovasc Surg 2015;27:234-9. [Crossref] [PubMed]
- Stella F, Dolci G, Dell'Amore A, et al. Three-dimesional surgical simulation-guided navigation in thoracic surgery: a new approach to improve results in chest wall resection and reconstruction for malignant diseases. Interact Cardiovasc Thorac Surg 2014;18:7-12. [Crossref] [PubMed]
- Marulli G, Dell'Amore A, Calabrese F, et al. Safety and effectivenessof cadaveric allograft sternocondral replacement after sternectomy: a new tool for the reconstruction of anterior chest wall. Ann Thorac Surg 2017;103:898-905. [Crossref] [PubMed]
- Rowsell AR, Eisenberg N, Davies DM, et al. The anatomy of the thoracodorsal artery within the latissimus dorsi muscle. Br J Plast Surg 1986;39:206-9. [Crossref] [PubMed]
- Fisher J, Bostwick J 3rd, Powell RW, et al. Latissimus dorsi blood supply after thoracodorsal vessel division: the serratus collateral. Plast Reconstr Surg 1983;72:502-11. [Crossref] [PubMed]
- Menke H, Erkens M, Olbrisch RR. Evolving concepts in breast reconstruction with latissimus dorsi flaps: results and follow up of 121 consecutive patients. Ann Plast Surg 2001;47:107-14. [Crossref] [PubMed]
- Glassey N, Perks GB, McCulley SJ. A prospective assessment of shoulder morbidity and recovery time scales following latissimus dorsi breast reconstruction. Plast Reconstr Surg 2008;122:1334-40. [Crossref] [PubMed]
- Arnold PG, Pairolero PC. Use of pectoralis muscle flaps to repair defects of the anterior chest wall. Plast Reconstr Surg 1979;63:205. [Crossref] [PubMed]
- Nahai F, Morales L Jr, Bone DK, et al. Pectoralis major muscle turnover flaps for closure of the infected sternotomy wound with preservation of form and function. Plast Reconstr Surg 1982;70:471. [Crossref] [PubMed]
- Daigeler A, Simidjiiska-Belayaeva M, Drucke D, et al. The versatility of the pedicled vertical rectus abdominis myocutanous flap in oncologic patients. Langenbecks Arch Surg 2011;396:1271-9. [Crossref] [PubMed]
- Fischer S, Klinkenberg M, Behr B, et al. Comparison of donor-site morbidity and satisfaction between anterolateral thigh and parascapular free flaps in the same patient. J Reconstr Microsurg 2013;29:537-44. [Crossref] [PubMed]
- Jurkiewicz MJ, Arnold PG. The omentum: an account of its use in the reconstruction of the chest wall. Ann Surg 1977;185:548-54. [Crossref] [PubMed]
- Berthet JP, Caro AG, Solovei L, et al. Titanium implant failure after chest wall osteosynthesis. Ann Thorac Surg 2015;99:1945-52. [Crossref] [PubMed]
- Turna A, Kavakli K, Sapmaz E, et al. Reconstruction with a patient-specific titanium implant after a wide anterior chest wall resection. Interact Cardiovasc Thorac Surg 2014;18:234-6. [Crossref] [PubMed]
- Aragón J, Mendez IP. Dynamic 3D printed titanium copy prosthesis: a novel design for large chest wall resection and reconstruction. J Thorac Dis 2016;8:E385-9. [Crossref] [PubMed]
- Metcalfe AD, Ferguson MW. Tissue enginreering in the replacement of skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 2007;4:413-37. [Crossref] [PubMed]
- Tang H, Xu Z, Qin X, et al. Chest wall reconstruction in a canine model using polydioxanone mesh demineralized bome matrix and bone marrow stromal cells. Biomaterials 2009;30:3224-33. [Crossref] [PubMed]
Cite this article as: Sanna S, Brandolini J, Pardolesi A, Argnani D, Mengozzi M, Dell’Amore A, Solli P. Materials and techniques in chest wall reconstruction: a review. J Vis Surg 2017;3:95.


