Video-assisted thoracoscopic subsegmentectomy for small-sized pulmonary nodules
Introduction
In recent years, pulmonary segmentectomy has been widely performed for small-sized pulmonary nodules (1). However, segmentectomy has some technical difficulties compared to that of lobectomy because the precise anatomical identification of pulmonary vessels and bronchi is required. For example, video-assisted thoracoscopic surgery (VATS) segmentectomy is more technically difficult to perform than an open thoracotomy. Furthermore, although techniques of subsegmentectomy in general are more complex, reports of VATS subsegmentectomy have been published more frequently due to an increase in the detection of smaller pulmonary nodules using developed computed tomography (CT) (2-4). In this article, we aimed to describe the techniques and roles of VATS subsegmentectomy.
The indication of VATS subsegmentectomy
The indication of limited lung resection for pulmonary nodules has still been unclear in recent years though limited resection for small-sized lung cancer with ground glass opacities (GGO) has been permissible. Asamura et al. stated that tumors <2 cm in diameter with a ratio of >75% GGO on radiography were pathologically noninvasive (5). Nakata et al. indicated that patients with tumors with GGO ratios >50% should be considered candidates for limited resection, although those with a GGO ratio of 50% exhibited vessel infiltration and experienced local recurrence after wedge resection (6). Limited resections include wedge resection, segmentectomy, and subsegmentectomy. Although the procedure of segmentectomy is generally thought to be more complex than a wedge resection, segmentectomy is a more anatomically detailed resection that can involve the dissection of hilar lymph nodes attached to the segment containing the tumor. Moreover, the oncological outcomes of segmentectomy in a propensity-matched study were comparable to those of lobectomy for patients with early-stage non-small cell lung cancer (6). Segmentectomy has thus become widely used worldwide (1). While wedge resection has been applied to smaller lung nodules located in peripheral parenchyma, segmentectomy has been applied to small-sized lung nodules that show not only GGOs, but also solid appearances located in deep parenchyma. Recent developed CT enabled the detection of small-sized pulmonary nodules containing GGO component. For such smaller pulmonary nodules, especially located in the deep parenchyma, subsegmentectomy may be an alternative procedure; this is because it is difficult to perform a wedge resection for hilum nodules located in the deep parenchyma and segmentectomy may be an excessive resection for appropriate surgical margins.
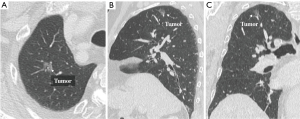
The role of subsegmentectomy is therefore considered as an intermediate procedure between wedge resection and segmentectomy. Consequently, we have concluded that the best indications for subsegmentectomy are as follows: (I) lung tumors of indeterminate nature, but considered suspicious for malignancy; (II) planned resection of a cT1aN0M0 primary lung cancer tumor less than 2 cm in diameter, with a GGO ratio of greater than 80% by high-resolution CT, in patients with good pulmonary function who are able to tolerate lobectomy (Figure 1A,B,C); (III) compromised resection in patients who are considered to be poor candidates for lobectomy due to limited cardiopulmonary reserve or other organ failure; (IV) the presence of metastases; and (V) a pathological diagnosis of benign tumors. Basically, we recommend indicating subsegmentectomy in cases for which wedge resection will be inappropriate due to the tumor size or its location deep in the parenchyma.
Technical aspects of VATS subsegmentectomy
An important process of segmentectomy is the identification of the intersegmental line. In previous papers, some methods of identifying the intersegmental line were reported. The conventional method involves creating an inflation-deflation line, a process which entails some troublesome issues. Okada and colleagues visualized the intersegmental line by selectively inflating the segment using a jet ventilator and reported this approach to be effective in securing an operative field (7). Expansion of the affected segment allows visualization of intersegmental borders while maintaining the morphology and size of the resected lung in the same state as the actual systemic physiological state, thereby achieving more accurate evaluation of resection margins. Therefore, since jet ventilation is deemed useful as an inflation method for the affected segment, it is becoming increasingly viewed as an advantageous method in Japan, worthy of standard use. However, this method requires equipment and another clinician to maneuver the bronchoscope. Because of these difficulties, various modifications have been reported. Direct air inflation and the injection of methylene blue or indocyanine green stain into the bronchus using a puncture needle inserted from the operative field were reported to be useful (8-11). However, great care is essential as this approach can reportedly cause air embolism (12). In subsegmentectomy, it is necessary for the surgeon to perform the more selective injection of air or dye into the subsegmental bronchus because the subsegmental bronchus is thinner than the segmental bronchus. Therefore, since it is difficult to create the intersubsegmental line, we initially applied the slip-knot method for VATS subsegmentectomy in 2010 (13). The slip-knot method does not require any special devices, such as jet ventilation, puncture needles, or injection of a dye solution into the targeted subsegmental bronchus, and it does not demand any other techniques, such as bronchoscopy or puncture needle. The essential device used in this method is simply a slip-knot made from a monofilament suture, and the essential technique involves pulling the slip-knot made outside the thoracic cavity after bilateral lung ventilation. Therefore, we consider our method is simpler, easier, and less expensive than any other conventional methods.
Another essential tool in VATS subsegmentectomy is three-dimensional CT (3-D CT) simulation (Figure 2). 3-D CT simulation provides useful information for thoracoscopic surgery (15,16). We previously reported on port-access thoracoscopic anatomic lung segmentectomy and subsegmentectomy using 3-D CT simulation for small lung nodules (17,18). The use of traditional CT is rapidly being replaced by multi-detector CT to facilitate visualization of the anatomy on the basis of 3-D imaging. Therefore, there are some published papers that report the use of 3-D CT to view pulmonary vasculature and parenchymal reconstruction preoperatively (17-20). Precise identification and division of the appropriate subsegmental veins is required for VATS subsegmentectomy. 3-D CT simulation can facilitate the accurate identification of these veins both preoperatively and intraoperatively. The identification of intersubsegmental veins and intrasubsegmental arteries and veins by 3-D CT simulation allows surgeons to remove the targeted subsegments.

Thus, using the slip-knot method and 3-D CT simulation, it is possible to perform VATS subsegmentectomy for small-sized lung tumors (Figure 3).

Roles of VATS subsegmentectomy
Subsegmentectomy is considered to be an anatomical resection compared to wedge resection, and a smaller volume resection than segmentectomy. There have been few reports about the role of subsegmentectomy. Nakamoto and colleagues described that subsegmentectomy reduces the loss of pulmonary tissue volume and is therefore considered to be more appropriate for smaller nodules (22). Furthermore, securing surgical margins is a very important factor in curative resection. Anatomical segmentectomy has been reported to be superior to wedge resection for securing adequate surgical margins (23). We have sometimes detected smaller lung nodules located near the intersegmental line in the hilum. In these limited resections, it is important to obtain sufficient surgical margins in order to secure a malignant tumor (24). However, wedge resection or uni-segmentectomy is sometimes insufficient to obtain surgical margins in the case of small-sized tumors located in the pulmonary hilum and near the intersegmental line. In such cases, an extended subsegmentectomy (bi- and tri-subsegmentectomy) or a segmentectomy combined with adjacent subsegmentectomy are preferred to wedge resection or uni-segmentectomy. Therefore, subsegmentectomy has been increasingly applied based on our technique of segmentectomy under 3-D CT simulation that we previously reported (17,18).
On the other hand, with the recently developed thin-section CT, we also have been able to encounter cases that involve smaller lung nodules located in slightly deeper parenchyma. In such cases, where tumors were usually non-visible and non-palpable, preoperative tumor marking is needed to detect the tumor location intraoperatively because intraoperative tumor detection is very difficult. Because of its associated challenges, the methods of intraoperative tumor detection have therefore been increasingly discussed in recent years. Although lobectomy or segmentectomy may completely resect such smaller nodules without any tumor markings because it is apparent in preoperative CT that the targeted small-sized lung nodules are contained in lobes or segments planned resections, it is assumed that these resections sometimes may lose more pulmonary volume than wedge resection and may be an excessive resection for small-sized pulmonary nodules. The number of resected subsegments in subsegmentectomy is smaller than in lobectomy or segmentectomy. Furthermore, it is assumed that wedge resection may be insufficient to secure surgical margins in small-sized tumors located in the deep parenchyma. Therefore, subsegmentectomy might preserve pulmonary function compared to lobectomy or segmentectomy, secure the sufficient surgical margin compared to wedge resection, and become an appropriate procedure for such tumors, thus demanding performance under the umbrella of thoracoscopic surgery in the future.
Our current original data and future prospects for VATS subsegmentectomy
In order to evaluate technical aspects of VATS subsegmentectomy, we reviewed clinical data from a consecutive series of 94 patients who underwent VATS subsegmentectomy and segmentectomy combined with adjacent subsegmentectomy between November 2006 and December 2016. Among the 94 patients, 52 underwent subsegmentectomy and 42 underwent subsegmentectomy combined with adjacent subsegmentectomy. Prior to 2010, the conventional method was used for creating the intersubsegmental plane during thoracoscopic surgery in 16 patients. From August 2010 onward, we applied the slip-knot method for creating an intersubsegmental plane during thoracoscopic subsegmentectomy. The slip-knot method was attempted in 66 patients. In an additional 10 patients treated from August 2010 and onward, we used conventional methods. Pulmonary subsegments and segments combined with adjacent subsegments from various locations were completely resected. The ratio of the accurate creation of an intersubsegmental line was 84.8%. Surgical time and bleeding volume were both reduced significantly after the introduction of this method.
Furthermore, we assessed the accurate resection rate of tumors deemed undetectable during VATS subsegmentectomy. Thirty-five patients were enrolled with non-palpable lung nodules, on whom VATS subsegmentectomy without any preoperative tumor markings was performed, and the impact of the 3-D CT simulation in the accurate resection was analyzed. Among 35 patients, 34 patients had tumors contained within the thoracoscopically resected subsegments (accurate resection rate of 97.1%). It was impossible to recognize the targeted tumor in the resected segment in only one patient, due to technical challenges associated with anomalies of the pulmonary vessels and bronchi, and due to the insufficient collapse of the lung associated with emphysema. We continued to perform the segmentectomy on the patient so the targeted tumor could finally be resected. None of the patients with lung cancer had metastasis of the hilar lymph nodes. Seven patients underwent an additional resection in which a stapler was used to secure an adequate margin because the original surgical margin was smaller than the size of the tumor (<2 cm).
Future prospect of instruments in VATS subsegmentectomy
Working space is limited during VATS. Moreover, the recent performance of VATS has trended toward reduced-ports surgery. Therefore, some instruments and devices have crucial roles for the successful completion of VATS. As new models of VATS devices develop, it is expected that new devices will be applied to subsegmentectomy because the subsegmental vessels and bronchi are very thin and a conventional stapler is too large for use in subsegmentectomy. A recent paper described the usefulness of energy devices or an endostapler (25-27). In particular, one new device called the MicroCutter has a narrower shaft than that of other conventional endostaplers and a 5 mm stapler. It is lighter, and is designed to function with more angulation, which allows surgery through a smaller incision with a larger degree of freedom. Such new devices will be useful, and the improvement of these devices will increase the demand for the performance of thoracoscopic anatomical resection of smaller nodules in the future.
In conclusion, VATS subsegmentectomy can be safely and accurately performed for small-sized lung tumors using the slip-knot method and 3-D CT simulation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Kanzaki M, Maeda H, Wachi N, et al. Complete video-assisted thoracoscopic multi-subsegmentectomy based on patients’ specific virtual 3-D pulmonary models. Asian J Endosc Surg 2013;6:110-5. [Crossref] [PubMed]
- Wu WB, Xu XF, Wen W, et al. Three-dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis 2016;8:S710-715. [Crossref] [PubMed]
- Nakayama H, Yamada K, Saito H, et al. Sublobar resection for patients with peripheral small adenocarcinomas of the lung: surgical outcome is associated with features on computed tomographic imaging. Ann Thorac Surg 2007;84:1675-9. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: Survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Nakata M, Sawada S, Yamashita M, et al. Objective radiologic analysis of ground-glass opacity aimed at curative limited resection for small peripheral non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;129:1226-31. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: Selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Zhang Z, Liao Y, Ai B, et al. Methylene blue staining: A new technique for identifying intersegmental planes in anatomic segmentectomy. Ann Thorac Surg. 2015;99:238-42. [Crossref] [PubMed]
- Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Kamiyoshihara M, Kakegawa S, Ibe T, et al. Butterfly-needle video-assisted thoracoscopic segmentectomy: a retrospective review and technique in detail. Innovations (Phila) 2009;4:326-30. [Crossref] [PubMed]
- Otsuka T, Nakamura Y, Harada A, et al. Extremely rare but potential complication of diffuse brain edema due to air embolism during lung segmentectomy with selected segmental inflation technique by syringe needle during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2011;142:e151-2. [Crossref] [PubMed]
- Oizumi H, Kato H, Endoh M, et al. Slip knot bronchial ligation method for thoracoscopic lung segmentectomy. Ann Thorac Surg 2014;97:1456-8. [Crossref] [PubMed]
- Kato H, Oizumi H, Suzuki J, et al. 3-D CT simulation (3-D CT angiography) for VATS subsegmentectomy of the left lower lobe, superior posterior subsegment. Asvide 2017;4:313. Available online: http://www.asvide.com/articles/1625
- Akiba T. Utility of three-dimensional computed tomography in general thoracic surgery. Gen Thorac Cardiovasc Surg 2013;61:676-84. [Crossref] [PubMed]
- Ikeda N, Yoshimura A, Hagiwara M, et al. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg 2013;19:1-5. [Crossref] [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomical thoracoscopic pulmonary segmentectomy under three-dimensional multi-detector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
- Kato H, Oizumi H, Inoue T, et al. Port-access thoracoscopic anatomical lung subsegmentectomy. Interact Cardiovasc Thorac Surg 2013;16:824-9. [Crossref] [PubMed]
- Tane S, Ohno Y, Hokka D, et al. The efficacy of 320-detector row computed tomography for the assessment of preoperative pulmonary vasculature of candidates for pulmonary segmentectomy. Interact Cardiovasc Thorac Surg 2013;17:974-80. [Crossref] [PubMed]
- Chan EG, Landreneau JR, Schuchert MJ, et al. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:523-8. [Crossref] [PubMed]
- Kato H, Oizumi H, Suzuki J, et al. VATS subsegmentectomy of the left lower lobe, superior posterior subsegment using the slip-knot method and 3-D CT simulation. Asvide 2017;4:314. Available online: http://www.asvide.com/articles/1626
- Nakamoto K, Omori K, Nezu K, et al. Superselective segmentectomy for deep and small pulmonary nodules under the guidance of three-dimensional reconstructed computed tomographic angiography. Ann Thorac Surg 2010;89:877-83. [Crossref] [PubMed]
- El-Sherif A, Femando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. [Crossref] [PubMed]
- Sawabata N. Locoregional recurrence after pulmonary sublobar resection of non-small cell lung cancer: can it be reduced by considering cancer cells at the surgical margin? Gen Thorac Cardiovasc Surg 2013;61:9-16. [Crossref] [PubMed]
- Özyurtkan MO, Kaba E, Toker A. Technological innovation in video-assisted thoracic surgery. J Vis Surg 2017;3:20. [Crossref]
- Gonzalez-Rivas D, Yang Y, Ng C. Advances in Uniportal Video-Assisted Thoracoscopic Surgery. Thoracic Surgery Clinics 2016;26:187-201. [Crossref] [PubMed]
- Schuchert MJ, Abbas G, Landreneau JP, et al. Usenof energy-based coagulative fusion technology and lung sealants during anatomic lung resection. J Thorac Cardiovasc Surg 2012;144:S48-51. [Crossref] [PubMed]
Cite this article as: Kato H, Oizumi H, Suzuki J, Hamada A, Watarai H, Nakahashi K, Sadahiro M. Video-assisted thoracoscopic subsegmentectomy for small-sized pulmonary nodules. J Vis Surg 2017;3:105.


