Robotic lobectomies: when and why?
According to NCCN guidelines (version 5.2017) regarding non-small cell lung cancer (NSCLC) “VATS or minimally invasive surgery (including robotic-assisted approaches) should be strongly considered for patients with no anatomic or surgical contraindications, as long as there is no compromise of standard oncologic and dissection principles of thoracic surgery” (1).
Throughout the course of the last decade, the role of minimally invasive surgery in thoracic surgery has been increasing. Since 1992 when Lewis et al. (2) firstly reported the use of video-assisted surgery to perform lobectomies, many changes have occurred to the thoracic approach to make surgery less invasive. Although the clear benefits versus open approach (less trauma, pain, and shorter hospital stay), video assisted thoracic surgery (VATS) has some limitations for the surgeon: bidimensional vision, camera under assistant’s control, long instruments in fixed ports, which create a fulcrum effect, and lack of tactile feedback.
Robot technology is an evolution of VATS, developed to overcome the restrictions of manual videothoracoscopy, maintaining the advantages related to low invasiveness.
The robotic system consists of a master console used by the surgeon to manipulate the patient cart, connected via electrical cables and optic fibres with three instrumental arms and a camera arm. The surgeon’s movements are transmitted to the cart manipulating master handles with a highly sensitive sensor able to filter physiologic hands tremor (6-Hz motion filter). The 3D high definition camera gives to the surgeon a much-improved vision compered to VATS and open approach. The robotic instruments, thanks to the seven degrees of freedom, allow the replication of the human wrist movement into the chest cavity. The three degrees of movement (pitch, yaw and insertion) are given by the cart arm; four degrees (internal pitch, internal yaw, rotation and grip) are guaranteed by the tip of the instrument, called in fact EndoWrist (3).
The da Vinci system® (Sunnyvale, CA, USA) is currently considered the only complete surgical system to perform thoracic surgery (4). During the years, four different generations of the robotic system have been developed (Standard, S, Si and Xi) with several improvements of the technological features, allowing feasible and safe surgical procedures.
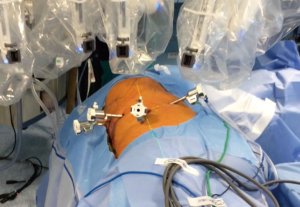
The last platform, Da Vinci Xi, is an important evolution of the previous systems. Significant improvements are centered on the patient cart features and on the docking process. The patient cart is a mobile platform with a boom-mounted system, easier to move than the prior systems. On the boom, there is a laser crosshair facilitating the alignment of the patient cart with the camera port (Figure 1). The patient cart can be placed in any position around the patient. The camera is smaller than the one in the previous systems and fits into an 8 mm trocar, which allows a port-to-port change of position. The Xi platform has also a laser targeting system, which assists with the alignment of the cart to target anatomy and to limit the arms collisions, frequent in the previous systems. All Xi instruments have longer shafts and the distance between robotic arms can be less than in the Si (6 vs. 8 cm). The robotic arms have an additional joint (patient clearance) which allows rotating away and avoids the collision with the patient’s body or with the other arms. Da Vinci Xi is provided with robotic staplers that allows performing a totally robotic lung resection without external positioning stapler under bidimensional vision and which avoids traumatisms during the manual staplers insertion through intercostal space.
Why?
Despite the profound changes and improvements that have taken place during the years and the increasing use of robotic system worldwide, the controversy about the application of RATS for lung resection is still open.
A drawback reported by most surgeons is the longer operating times: the robotic time to perform a lobectomy is averagely longer than that of an open or a VATS approach.
The average times reported by more experienced robotic surgeon are between 100 and 228 min (5-12). Anyway, the introduction of the Xi system has sensibly decreased the mean operative time of robotic procedures, thanks to shorter docking time and to technological improvements of the platform.
In our opinion, an important mean to decrease surgical time is the standardization of the surgical technique, firstly the port mapping: a mistake during this point could complicate the identification and the proper isolation of hilar structures with a longer operative time. Different authors describe several techniques in regard. Park et al. (13) described a three robotic arms technique with two thoracoscopic ports and a 4 cm utility incision. Gharagozloo et al. (6) reported a hybrid technique with three robotic arms, (positioned at the 8th, camera, 6th and 5th intercostal space), in this case the surgeon used a robotic approach for hilar structures dissection, then the platform was removed and he returned to the operating table to complete the operation. Louie et al. (11) and Anderson et al. (14) described a three-arm robotic lobectomy with a utility port; Jang et al. (15) used a utility incision at the fifth intercostal space. Ninan and Dylewski (16) reported a three arms technique using the same intercostal space for all ports (the 5th or 6th) and a utility port over the 11th rib. Veronesi et al. (7) and Cerfolio et al. (10) described four arms robotic lobectomy without utility incision.
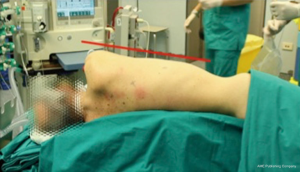
At the Robotic Surgery Unit in Pisa we are currently using a four arms technique without utility incision. The patient is positioned in lateral decubitus, as for a posterior-lateral thoracotomy, with the operating table tilted at the tip of the scapula (Figure 2). When using Si platform the camera port (10 mm) is positioned in the 7th or 8th intercostal space on the mid axillary line; the other ports (8 mm) are positioned in the 5th or 6th intercostal space on the anterior axillary line, in the 6th or 7th intercostal space on the posterior axillary line and in the auscultatory area. Recently, thanks to the introduction of the robotic staplers in the Xi platform, we have modified our port mapping. The posterior ports are positioned (when possible, depending on the chest dimension) along the same intercostal space (7–8th intercostal space) and in the auscultatory area (between the posterior rime of the scapula and the spine). The anterior port is positioned in the 5–6th intercostal space on the anterior axillary line, just over the diaphragm (Figure 3). Considering the variability of the chest dimensions, however, it is highly recommended to check the position of the port through the internal camera view, in order to perform the higher posterior access at the level of posterior inter-lobar fissure line. The Xi port mapping modification simplifies the stapler movements and allowing the positioning of all the posterior access in the same intercostal space, reducing postoperative pain. Thanks to our long experience with all the generations of robotic platforms, we have had the possibility to optimize the trocar position standardizing the procedure.
Currently, the instruments used during all major lung resections are: monopolar (e.g., Hook, Scissors) or bipolar instruments (e.g., Maryland) for dissection and graspers (e.g., Cadiere, Prograsp). The dissection of the hilar structures can be performed by the action of monopolar and/or bipolar instrument, while a grasper, inserted through the fourth arm, is used to retract the lung obtaining optimal exposition of the mediastinum. During the surgical procedure, CO2 is insufflated (range, 5–8 mmHg) to drive the diaphragm down, enlarge the chest cavity and guarantee a good exposition of hilar structures.
Another aspect to take into account is the learning curve (the process of gaining experience and developing skills to make a procedure) of robotic surgery, some authors affirm that is shorter than that needed for traditional videothoracoscopic surgery. Gharagozloo et al. (6), Veronesi et al. (17) and Melfi et al. (18) suggested a learning curve of 20 robotic lobectomies for an experienced thoracic surgeon. Several studies suggest a wide range of cases (50 and 100/200) (19,20) to achieve a yield in VATS lobectomy. This difference between the Robotic and the VATS learning curves is likely to be due to the particular features of the robotic system that allow to perform the surgical procedures with the same approach and timing of the open surgery.
In our opinion it is mandatory to start the learning process with simple procedures such as for example, mediastinal lesions removal, and then to continue to more complex surgical interventions, such as lobectomies.
A further criticism raised against robotic surgery is the little data available about oncological radicality and survival with adequate follow-up period.
Indirect indicators of oncological radicality generally used are the number of lymph nodes resected and the lymph-nodal upstaging (the capacity to histopathologically identify metastatic lymph nodes clinically staged as negative), moreover, an adequate lymphadenectomy is essential to prevent under staging, with consequent lack of adjuvant treatment and worsening of prognosis. Nodal staging is therefore a surrogate of the quality of surgery. Two recent papers have shown a cutting point of 16 examined lymph nodes in the evaluation of the quality of LN examination or prognostic stratification postoperatively for patients with declared node-negative disease (21,22).
Discordant data exists on the radicality of nodal harvest during VATS lobectomies, more frequently, in fact, a lower median number of dissected lymph nodes are found and fewer nodal upstaging, particularly for the N2 group, when compared to open surgery (23-25).
Several authors reported their experience on analysis of lymph nodal upstaging in VATS procedures and thoracotomy resections, in most of the cases the studies reported a lower rate of upstaging in VATS group. D’Amico evaluated 189 patients underwent open lobectomy and 199 VATS lobectomy and observed different upstaging to N1/N2 between the two groups: 14.5% cases in the open group and 8.8% in VATS one (26). Also, Licht analysing 1,513 lobectomies for clinical stage I NSCLC performed by VATS or open surgery, confirms lower upstaging in VATS group than thoracotomy group (11.9% vs. 24.6%), although the mean number of dissected lymph nodes stations were similar and no difference in survival was showed between two groups (27).
Boffa and colleagues conversely in a report of 11,500 anatomic lung cancer resection from the Society of Thoracic Surgeon database showed a similar lymph nodal upstaging after VATS and open surgery (11.6% vs. 14.3%) (28).
Comparing lymph nodal dissection by VATS versus open surgery a critical aspect is the evidence of superior number of mediastinal nodes removed during thoracotomy procedures, probably due to the greater difficulty to reach comfortably all mediastinal areas with thoracoscopic instruments (29).
The dissimilar results between VATS and thoracotomy lymphadenectomy in comparative studies are probably related with different expertise and level of skills of the surgeons.
Conversely, several studies have demonstrated the equivalence between robotic and open nodal dissection. According to these studies, the median number of lymph nodes resected with robotic approach is the same of open surgeries (17,30).
In our opinion, the robotic approach gives also a better dissection than VATS in a confined space of enlarged N1 lymph nodes and a more precise N2 lymph nodes removal.
Wilson reported the first experience of upstaging in patients with clinical stage I NSCLC who underwent robotic lobectomies. In this study upstaging was observed in 10.9% of cases, especially in patients with larger lung tumor (31). Park reports a 21% rate of nodal upstaging (6,32) and Velez-Cubian et al. a 30% of overall upstaging rate (33).
Despite the controversy over lymphadenectomy data, the long term survival and disease free survival are similar in NSCLC treated by VATS and open surgery, confirming the effectiveness of the mini-invasive procedure (29,31,34,35). The outcomes oncologic results in robotic treatment for lung cancer are more recent than VATS, not many large studies on long-terms outcomes have been reported. Park shows an overall 5-year survival of 80% (32), Wilson a 2-year overall survival of 87.6% with a DFS of 70.2% (31) and Melfi a 5-year actuarial survival of 80% (36).
However, the most criticized aspect is represented by costs of robotic platforms. Several studies have been carried out to compare the costs of VATS, thoracotomy and Robotic procedures. In 2008, Park and Flores (37) conducted a retrospective review to determine the expenses associated with the resultant hospital stay. The authors found robotic procedures less expensive than thoracotomy ($4,380 vs. $8,368), but more costly than VATS ($1,479).
Cost control is a fundamental aspect for a healthcare system, and for this very reason in Pisa was created a multidisciplinary robotic centre. In order to minimize costs, the managerial strategy of our centre is based on high surgical volumes, complex procedures and standardization of the technique. After 6 years of experience, with the increasing number of the robotic procedures and thanks to the standardization of the technique (prefixed instruments, shorter docking time, dedicated team of surgeons, anaesthetists and scrub nurses) the centre has obtained a positive result: robotic surgery has been actually considered revenues from disease-related-groups (DRGs) (38).
When?
With regard to the indications of the robotic approach, we noticed that in the majority of cases robotic lung resection is offered to very selected patients, with early clinical stages (I and II) and no comorbidities, some authors also add dimensional criteria and exclude the lesions that are greater than 5 cm (18). Recently some authors have extended the inclusion criteria and have treated with a robotic approach patients with advanced stages, as clinical IIIA stage after neoadjuvant therapies (39), or have performed sleeve lobectomy or robotic bronchoplastic upper lobectomy (40).
A review conducted by Kent et al. (41) collecting data from 33,095 patients treated with open, VATS and robotic approach in eight countries between 2008 and 2010, has shown that in “high-volume surgeons” robotic lobectomy is associated with a reduction in mortality, length of stay and overall complication rate compared with thoracotomy. Robotic lobectomy is also associated with a statistically significant reduction in mortality compared with VATS lobectomy.
As regards as the quality of life (QoL), Cerfolio et al. (30) firstly reported an analysis on thoracic robotic surgery patients that shows a significantly higher average mental QoL score 3 weeks postoperatively compared to open surgery.
Louie and colleagues (11) declared that patients operated with robotic-assisted surgery used fewer painkillers and returned to daily life sooner than when compared with VATS. A recent study by Kwon et al. (42) has shown no significant difference in acute and chronic postoperative pain between VATS and RATS. Interestingly patients who underwent robotic surgery felt that the robotic approach affected positively their pain, indicating an important difference between real and perceived pain.
A recent study published by Park et al. (43) compared post-operative data and survival outcomes of patients treated with lobectomy after induction chemotherapy using minimally invasive approaches (VATS or robotic procedures) or thoracotomy. The results show a similar OS and DFS between the two groups, suggesting the feasibility of using minimally invasive approaches, following induction therapy, to treat selected locally advanced stages of NSCLC.
Doubtlessly, the use of robotic system in thoracic surgery is still evolving as well as its indications and applications.
Moreover, several studies suggest that perioperative outcomes, including postoperative complications, are similar between robotic and conventional surgery (44).
Conclusions
Robotic surgery for lung lobectomy is feasible, safe, provides several improvements both for the patient (mainly in terms of higher rates of lymph nodal upstaging with less operative morbidity) and for the surgeon (advanced features of robotic platform and reduced learning curve) when compared to open and VATS approach in specialized centres (45).
Regarding the most discussed aspect of robotic procedures, its high capital and running costs, we believe that a management’s strategy based on high surgical volumes, complex procedures and standardization of technique could reduce the costs of robotic procedures.
Therefore, taking into account what has been said, the right question to ask should be: “why not”?
Acknowledgements
We thank Teresa Hung Key for linguistic accuracy checking.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- NCCN Non-small-cell-lung-cancer guidelines. Version 5.2017. [cited 2017 Mar 03].
- Lewis RJ, Caccavale RJ, Bocage JP, et al. Video-assisted thoracic surgical non-rib spreading simultaneously stapled lobectomy: a more patient-friendly oncologic resection. Chest 1999;116:1119-24. [Crossref] [PubMed]
- Ambrogi MC, Fanucchi O, Melfi F, et al. Robotic surgery for lung cancer. Korean J Thorac Cardiovasc Surg 2014;47:201-10. [Crossref] [PubMed]
- Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Augustin F, Bodner J, Wykypiel H, et al. Initial experience with robotic lung lobectomy: report of two different approaches. Surg Endosc 2011;25:108-13. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg 2011;91:1729-36; discussion 1736-7.
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Davini F, et al. Robotic lobectomy for lung cancer: evolution in technique and technology. Eur J Cardiothorac Surg 2014;46:626-30; discussion 630-1. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Anderson CA, Hellan M, Falebella A, et al. Robotic-assisted lung resection for malignant disease. Innovations (Phila) 2007;2:254-8. [Crossref] [PubMed]
- Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. [Crossref] [PubMed]
- Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]
- Veronesi G, Agoglia BG, Melfi F, et al. Experience with robotic lobectomy for lung cancer. Innovations (Phila) 2011;6:355-60. [Crossref] [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning thoracoscopic lobectomy. Eur J Cardiothorac Surg 2010;37:516-20. [Crossref] [PubMed]
- Li X, Wang J, Ferguson MK. Competence versus mastery: the time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2014;147:1150-4. [Crossref] [PubMed]
- Becker DJ, Levy BP, Gold HT, et al. Influence of Extent of Lymph Node Evaluation on Survival for Pathologically Lymph Node Negative Non-Small Cell Lung Cancer. Am J Clin Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Martin JT, Durbin EB, Chen L, et al. Nodal Upstaging During Lung Cancer Resection Is Associated With Surgical Approach. Ann Thorac Surg 2016;101:238-44; discussion 44-5. [Crossref] [PubMed]
- Medbery RL, Gillespie TW, Liu Y, et al. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2016;11:222-33. [Crossref] [PubMed]
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [Crossref] [PubMed]
- D'Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31; discussion 231-2. [Crossref] [PubMed]
- Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010;89:1730-5; discussion 1736.
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7.
- Park BJ. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg 2012;1:24-6. [PubMed]
- Velez-Cubian FO, Rodriguez KL, Thau MR, et al. Efficacy of lymph node dissection during robotic-assisted lobectomy for non-small cell lung cancer: retrospective review of 159 consecutive cases. J Thorac Dis 2016;8:2454-63. [Crossref] [PubMed]
- Berry MF, D'Amico TA, Onaitis MW, et al. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. Ann Thorac Surg 2014;98:197-202. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 960-1. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Davini F, et al. VATS-based approach for robotic lobectomy. Thorac Surg Clin 2014;24:143-9. v. [Crossref] [PubMed]
- Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 2008;18:297-300. vii. [Crossref] [PubMed]
- Veronesi G, Cerfolio R, Cingolani R, et al. Report on First International Workshop on Robotic Surgery in Thoracic Oncology. Front Oncol 2016;6:214. [Crossref] [PubMed]
- Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y, Miwa K, et al. A successful case of robotic bronchoplastic lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2013;19:478-80. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Kwon ST, Zhao L, Reddy RM, et al. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Park BJ, Yang HX, Woo KM, et al. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:S406-13. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Ricciardi S, Cardillo G, Zirafa CC, Davini F, Melfi F. Robotic lobectomies: when and why? J Vis Surg 2017;3:112.