Endobronchial thermoplasty for asthma
Introduction
Asthma is an incurable chronic disease affecting approximately 24 million people in the United States. The hallmark features of asthma are reversible airflow obstruction, airway hyperresponsiveness, airway inflammation, bronchoconstriction, and excessive mucus secretion. Clinical symptoms include episodic or persistent breathlessness, wheezing, cough, or chest tightness/pressure. Forty-five percent of asthmatics continue to have yearly exacerbations and the disease is responsible for approximately 3,600 annual deaths (1). Asthma severity and control can have major impacts on healthcare and personal expenses related to asthma (2,3).
Pharmacologic treatment modalities in a “step-wise” fashion have long been the mainstay of treatment. The enhancing knowledge on different asthma phenotypes will undoubtedly lead to more targeted and patient specific pharmacologic agents. Despite improvement in drugs there continues to be a subset of asthmatics less responsive to current therapies. This creates opportunities for more innovative approaches. Bronchial thermoplasty (BT) is a procedural based treatment utilizing radiofrequency (RF) ablation of airway smooth muscle (ASM).
Premise of BT
The principle of BT is based on the utility and role of ASM. Asthmatic airways undergo remodeling which leads to structural and functional changes (4). One of many changes involved in this remodeling process is hypertrophy and hyperplasia of ASM (5,6). The role of ASM in the healthy lung is incompletely understood and some have argued that if it were eliminated there would be no physiologic consequence, deeming it a vestigial organ (6). Though the role in the healthy lung is incompletely understood, its pathologic role in asthma has been well demonstrated. The integrity of ASM is particularly sensitive to heat. BT utilizes a highly controlled heat delivery system with the use of RF energy generation to disrupt ASM. The disrupted tissue is replaced by loose connective tissue, effectively decreasing ASM bulk. This leads to less hyperresponsiveness, bronchoconstriction, and interaction with the extracellular matrix (ECM).
Equipment and procedure
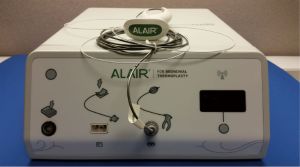
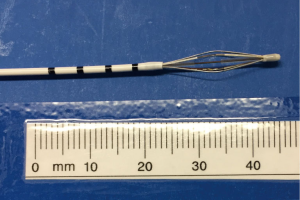
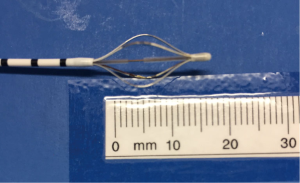
The Alair Bronchial Thermoplasty System (Asthmatx Inc., Sunnyvale, CA, USA) is a compact system comprised of the Alair Radiofrequency Controller, disposable single-use catheter, foot pedal, and gel-type patient return electrode (Figure 1). The distal end of the 1.4 mm diameter catheter has a basket-like array of expandable electrodes. The basket-like arrays can expand to a maximum of 13 mm (Figures 2,3). The catheter is designed to be used in a flexible bronchoscope with a 2.0 mm working channel. Avoidance of larger diameter bronchoscopes allows for better visualization of the distal bronchial subsegments and thus increases the potential treatable airways.
Patients with uncontrolled severe persistent asthma should be clinically stable at the time of the proposed procedure (see indications and contraindications). Prednisone at a dose of 50 mg per day is started 3 days prior to the procedure and continued until the day following the procedure. The procedure is performed over 3 sessions with at least a 3-week interval between sessions. Each session treats a different area from the right lower lobe, left lower lobe, and finally the bilateral upper lobes. By convention, the right middle lobe is not treated secondary to the narrow orifice.
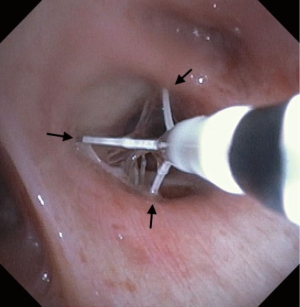
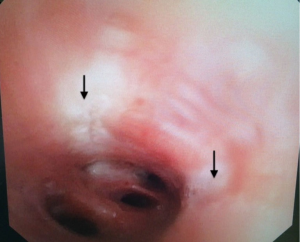
Pre-procedural spirometry with documentation of FEV1 is recommended. Next, the patient is sedated with either conscious sedation or general anesthesia. The bronchoscope is then introduced and the Alair Catheter is advanced through the working channel. The electrode array is advanced to the most distal airways within direct visualization or until slight resistance is felt. The catheter is then withdrawn approximately 1 cm. The array is then gently expanded to allow each electrode to have contact with the mucosa (Figure 4). A minimum of 3 arrays must have contact with the wall for a successful activation. The RF Controller is activated by the foot pedal and in approximately 10 seconds the airway is heated in a highly controlled manner to 65 °C. An audible alert serves as notification of a successful activation. If mucosal contact is compromised then there is an alternative audible alert and the activation is terminated. Following a successful activation, the catheter is withdrawn 5 mm (assisted by black markings on catheter) and the process is repeated until the entire area is treated. Please note that the last activation in the same segment would be the last black line closest to the arrays. It is not uncommon to see blanching on the mucosa where the arrays touch (Figure 5). Refrain from allowing the arrays to come in contact with the tip of the bronchoscope during activation to avoid any possible damage to the bronchoscope. The airways treated, the number of activations, and the number of failed activations should be documented.
Post-procedure care consists of monitoring for 2–4 hours and completion of post-procedure spirometry. The FEV1 should be within 80% of the pre-procedural FEV1. If the FEV1 is less than 80% pre-procedural FEV1 then the patient should remain in the hospital for further observation. After discharge the patient should be contacted 1, 2, and 7 days after the procedure for a clinical update. A follow up visit is recommended 3 weeks after the procedure to assess recovery and plan for the next session (Figures 6,7) (7,8).


Patient selection
As of 2014 the International ERS/ATS guidelines on definition, evaluation, and treatment of severe asthma recommended that if BT is performed it is performed in adults with severe asthma in the context of an Institutional Review Board approved independent systematic registry or a clinical study. These recommendations are primarily based on concerns of avoiding adverse effects until the optimal patient population can be further defined (9). The Global Strategy for Asthma Management and Prevention denote that BT can be used for highly-selected adult patients with uncontrolled asthma despite the use of recommended therapeutic regimens and referral to an asthma specialty center (10).
BT patients should be severe persistent asthmatics with uncontrolled symptoms who have been evaluated by a pulmonary specialist well versed in advanced asthma care. Medical regimen should include an inhaled corticosteroid (ICS) (>1,000 µg/d beclomethasone or equivalent), long acting beta agonist (LABA) (≥100 µg/d salmeterol or equivalent), and a short acting beta agonist (SABA).
The pulmonologist should extensively investigate the potential reasons for treatment failure including a thorough evaluation of an alternative diagnosis other than asthma.
Reversible airflow obstruction and hyperresponsiveness on provocation testing should be confirmed. Associated co-morbidities which may limit asthma symptom control should be optimized including postnasal drip, allergic rhinitis, nasal polyps, gastroesophageal reflux disease, obstructive sleep apnea, and medication non-compliance. Alternative diagnoses should include, but are not limited to, vocal cord dysfunction, chronic obstructive pulmonary disease, allergic bronchopulmonary aspergillosis, Churg-Strauss Syndrome, bronchiolitis obliterans, Granulomatosis with polyangiitis, excessive dynamic airway collapse, tracheobronchomalacia, drug reactions, interstitial lung disease, bronchiectasis, and uncontrolled cardiac etiologies. A reasonable evaluation should include a complete blood count with differential, basic metabolic panel, coagulation studies, high resolution computed tomography with inspiratory and expiratory views, comprehensive pulmonary function testing, provocation testing, serum IgE levels, skin Prick test, and allergy testing. Results from these tests can assist if any further evaluation is indicated.
Contraindications for BT include general contraindications to undergo flexible bronchoscopy, presence of a pacemaker, defibrillator, or other implantable device, previous treatment with BT, recent asthma exacerbation (typically within 6 weeks of planned procedure), recent respiratory tract infection, known coagulopathy, recent change in systemic steroid requirements, uncontrolled co-morbidities, a greater than 10-pack-year history of tobacco use or tobacco use within 1 year.
Review of pivotal studies in the development of BT
Initial investigations were performed by Danek and colleagues in 2004. A total of 11 dogs were treated with varying target RF induced temperatures of 55, 65, and 75 °C, and a control arm. Airway responsiveness to local methacholine challenge was documented based on airway diameter with a significant reduction in airway responsiveness in the 65 and 75 °C treatments groups which persisted for the 3 years of the study. No significant adverse events were noted.
Subsequently Miller and colleagues performed the first prospective feasibility trial on humans. This was an innovative study that recruited patients with planned lung resections for known or suspected malignancy. BT was performed up to 3 weeks prior to the planned resection which allowed for repeat bronchoscopy at the time of resection as well as anatomic specimens to study. Follow-up airway examination at the time of resection did demonstrate areas of airway edema and redness but no evidence of airway scaring and histologic examination confirmed reduction in ASM confined to the airway wall and peri-bronchial region (11).
The first BT study in asthmatic patients was performed in 2006 by Cox and colleagues. This was a nonrandomized prospective trial aimed at assessing the safety of BT in patients with mild to moderate asthma. It should be noted that the proposed population currently is for patients with severe persistent asthma. This was a small trial of 18 patients with 2 withdrawing prior to treatment. There were a total of 312 adverse events over a 2-year period ranging from mild (74%), moderate (25%), and severe (1%). One hundred and fifty-five of these events were considered device/procedure related with the mean onset at 1.7 days and resolution by 4.6 days. Longer-term safety assessment at 2 years showed no deterioration of FEV1 or overall respiratory health status. This was a pioneering study that demonstrated that BT could be safely performed in asthmatic patients (12).
The first randomized control trial was published in 2007 by Cox and colleagues. The Asthma Intervention Research (AIR) trial consisted of 112 patients on ICS and LABA who demonstrated impaired control upon withdrawal of their LABA. Primary outcome was to evaluate the frequency of mild exacerbations. Secondary outcomes included airflow, airway responsiveness, asthma symptoms, number of symptom-free days, use of rescue medications, Asthma Control Questionnaire (ACQ) score, and Asthma Quality of Life Questionnaire (AQLQ) score. Assessments were obtained at 3, 6, and 12 months. Those randomized to BT had a baseline mild exacerbation rate of 0.35±0.32 exacerbations per subject per week. Twelve months after treatment this reduced to 0.18±0.31. The control arm went from 0.28±0.31 to 0.31±0.46 exacerbations per subject per week. Secondary outcomes demonstrated an improvement in morning peak flow, a significant increase in the AQLQ (1.3±1.0 versus 0.6±1.1), a reduction in ACQ score (1.2±1.0 versus 0.5±1.0), and an increase in symptom free days (40.6±39.7 versus 17.0±37.9) (13) A clinically significant change in AQLQ and ACQ is 0.5 (14,15). The study did demonstrate an increase in adverse respiratory events in the BT group, generally within one day of the procedure with resolution by day 7.
Additional long-term safety data from the AIR trial was later published in 2011. Forty-five patients from the BT arm and 24 patients from the standard of care arm enrolled for long term follow up. BT patients were followed at year 2, 3, 4, and 5. Standard of care follow up occurred at year 2 and 3. LABA use was reduced in 57% of the BT subjects at 5 years compared to 54% of the standard of care arm at 3 years. There was no significant difference in reduction of ICS dose between the two groups. There was no significant decrease in FEV1 or FVC and follow up radiographic images showed no significant changes (16). This follow up study demonstrated more the lack of detrimental effects at 5 years out from BT.
The post hoc analysis from the AIR trial suggested that those with severe disease may benefit the most from BT. Further evaluation on the safety and efficacy of BT on those with severe asthma was investigated by Pavord and colleagues. The Research in Severe Asthma (RISA) trial enrolled 32 patients with severe asthma, BT (n=15) and control arm (n=17). The BT arm had a greater number of adverse events, 136 versus 57. Seven hospitalizations during the treatment period were required in the BT arm (4 patients) for asthma exacerbation (5) and lobar collapse (2). Medication use was compared during three phases, steroid stable phase (week 6–22), steroid wean phase (week 22–36), and reduced steroid phase (week 36–52). During the steroid stable phase SABA use was significant reduced in the BT arm compared to the control arm, −26.6±40.1 versus −1.5±11.7 puffs per week. The BT arm had significant improvement in the percentage change from baseline in pre-bronchodilator percent predicted FEV1, (14.9±17.4% versus −0.9±22.3%). AQLQ and ACQ scores also demonstrated clinically significant improvements during the steroid stable phase. Analysis at the time of reduced steroid phase continued to show a reduction in SABA use (−25.6±31.2 versus −6.1±12.4) and persistent improvement in ACQ score (−0.99±0.83 versus −0.22±0.78) but the improvement in percent predicted FEV1 and AQLQ did not persist (17).
A major limitation of the aforementioned trials was the possibility of a significant placebo effect given the interventional nature of the treatment. The AIR2 trial was a multicenter, randomized, double-blind, sham-controlled clinical trial aimed at evaluating the effectiveness of BT based on AQLQ scores in severe symptomatic asthmatics. There was randomization of 297 patients to BT (n=196) or sham procedure (n=101). Nine subjects withdrew consent and did not have a procedure performed whereas the remaining subjects all had at least one bronchoscopic procedure. Of this population, both groups demonstrated a clinically significant improvement in AQLQ. The BT groups had an improvement of 1.35±1.10 and the sham group had an improvement of 1.16±1.23. There was a greater than expected placebo effect on the mean change of AQLQ scores but the proportion of subjects that had a clinically significant improvement varied between the BT and sham groups, BT group (79%) versus sham group (63%). Only 3% of the BT group had a clinically significant deterioration in AQLQ score versus 7% of the sham group. The secondary outcomes showed a 32% reduction in severe exacerbation rate. There was improvement in morning peak expiratory flow, symptom free days, symptom score, ACQ score and reduced rescue medication use in both groups without a significant difference between them (18).
Wechsler and colleagues continued to assess the 5-year safety of BT in this population of severe symptomatic asthmatics. Of the 190 initial BT patients there was follow up of 162. When compared to pre-BT events, there was a 44% reduction in exacerbations and a 78% reduction in ER visits. As noted in previous studies, the FEV1 and imaging remained stable (19).
Future direction
The primary target of BT continues to be focused on ASM reduction. ASM likely has a multidimensional effect on asthma which continues to be studied and delineated. The interaction of ASM and the ECM likely plays a significant role in the evolution of asthma. Further investigations continue to be underway to understand this complex interaction. The role of BT in severe symptomatic asthma will continue to be defined but as of today should be utilized in those patients in which it has been shown to be safe and effective.
Conclusions
BT is a treatment modality very different from the traditional approach to the treatment of asthma. This innovative approach has brought on criticism and doubt but the large number of trials has proven it to be a safe and possibly effective tool in the treatment of asthma. As the medical community continues to better understand the asthma phenotype that will best respond to BT, we believe the effectiveness in improvement in quality of life and reduction in asthma related healthcare and personal costs will be reduced. BT is a feasible and promising option in the treatment of severe asthmatics with persistent symptoms.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Centers for Disease Control and Prevention. Most Recent Asthma Data. Available online: https://www.cdc.gov/asthma/most_recent_data.htm
- Godard P, Chanez P, Siraudin L, et al. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J 2002;19:61-7. [Crossref] [PubMed]
- Ivanova JI, Bergman R, Birnbaum HG, et al. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol 2012;129:1229-35. [Crossref] [PubMed]
- An SS, Bai TR, Bates JH, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J 2007;29:834-60. [Crossref] [PubMed]
- Vignola AM, Mirabella F, Costanzo G, et al. Airway remodeling in asthma. Chest 2003;123:417S-22S. [Crossref] [PubMed]
- Mitzner W. Airway smooth muscle: the appendix of the lung. Am J Respir Crit Care Med 2004;169:787-90. [Crossref] [PubMed]
- Zamora F, Cho R, Rao M, et al. Treatment of right lower lobe anterior-basal segment. Asvide 2017;4:380. Available online: http://www.asvide.com/articles/1694
- Zamora F, Cho R, Rao M, et al. Treatment of medial-basal segment of right lower lobe. Asvide 2017;4:381. Available online: http://www.asvide.com/articles/1695
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343-73. [Crossref] [PubMed]
- Global Strategy for Asthma Management and Prevention. Available online: http://ginasthma.org/wp-content/uploads/2016/04/wms-GINA-2016-main-report-final.pdf
- Miller JD, Cox G, Vincic L, et al. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005;127:1999-2006. [Crossref] [PubMed]
- Cox G, Miller JD, McWilliams A, et al. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006;173:965-9. [Crossref] [PubMed]
- Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007;356:1327-37. [Crossref] [PubMed]
- Juniper EF, Guyatt GH, Willan A, et al. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol 1994;47:81-7. [Crossref] [PubMed]
- Juniper EF, Svensson K, Mork AC, et al. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005;99:553-8. [Crossref] [PubMed]
- Thomson NC, Rubin AS, Niven RM, et al. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med 2011;11:8. [Crossref] [PubMed]
- Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007;176:1185-91. [Crossref] [PubMed]
- Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010;181:116-24. [Crossref] [PubMed]
- Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol 2013;132:1295-302. [Crossref] [PubMed]
Cite this article as: Zamora F, Cho R, Rao M, Gibson H, Dincer HE. Endobronchial thermoplasty for asthma. J Vis Surg 2017;3:127.