Endoscopic mucosal ablation and resection of Barrett’s esophagus and related diseases
Introduction
The normal esophageal mucosa is lined by a stratified non-keratinized squamous epithelium that provides, through tight cell junctions, an effective barrier that resists penetration by luminal particles (1). However, it has been shown that this stratified squamous epithelium has a lower resistance and lower protective effect when exposed to chronic gastroesophageal reflux, with the rapid development of epithelial changes progressing from reflux esophagitis, to columnar metaplasia of cardia type to eventually intestinal metaplasia (1,2). The evidence of intestinal metaplasia with one or more goblet cells are the microscopic findings that establishes the diagnosis of Barrett’s esophagus (BE) (Figure 1A) (3).
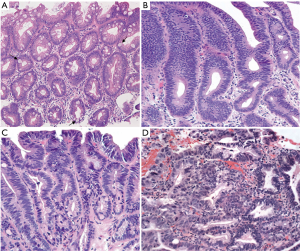
BE is considered a premalignant lesion that increases the risk of developing esophageal cancer, progressing from non-dysplastic intestinal metaplasia to low-grade dysplasia (LGD), high-grade dysplasia (HGD) and ultimately esophageal adenocarcinoma (EAC).
Although the progression rate from non-dysplastic BE to EAC is less than 1% per year (4,5), with management focused on proton pump inhibitors and endoscopic surveillance (6), the risk of progression from LGD to HGD and from HGD to EAC is considerably higher (4,7-9). This higher rate of progression to EAC highlights the importance of an early detection of dysplastic changes and the adoption of appropriate therapeutic approaches to prevent malignancy progression.
Endoscopic therapies like radiofrequency ablation (RFA) and endoscopic mucosal resection (EMR) are the preferred techniques for the management of patients with dysplastic BE and/or mucosal abnormalities suspicious of EAC (6,10). Both therapies have shown to be safe and effective in the eradication of dysplastic changes and in reducing the progression rate to EAC (11-14). Ablative therapies such as RFA are aimed to eliminate the dysplastic BE by causing necrosis of the esophageal mucosa. After satisfactory tissue ablation, the esophageal epithelium is replaced by normal squamous mucosa, or so called neo-squamous epithelium. Similarly, EMR is a technique used for the management of mucosal irregularities suspicious of malignancy with both diagnostic and therapeutic purposes (6).
Here, we will show the technique and utility of RFA and EMR in the treatment of dysplastic BE and intramucosal tumors.
Patient selection
An accurate and careful selection of patients for RFA and EMR is crucial to improve outcomes and avoid complications. Ideally, patients with dysplastic BE after tissue biopsy or patients with mucosal abnormalities suspicious for malignancy should be offered endoscopic therapies like RFA and EMR.
Patients with non-dysplastic BE are candidates for ablative therapies on a selective basis which may include long-segment BE (>3 cm), those with a family history of esophageal cancer or higher risk patients with poorly controlled reflux. Their management also focuses on proton pump inhibitors and endoscopic surveillance every three years (6).
Establishing the diagnosis of LGD can be difficult with high inter-observer variability (15). Consequently, it is recommended that the diagnosis of LGD should be confirmed by a second experienced pathologist (6). Once the diagnosis of LGD is confirmed (Figure 1B), these patients should be offered RFA as a treatment option, which has shown excellent results with low complication rates and a reduction in the rates of progression to HGD and EAC (10,14,16). As shown by Shaheen et al. (16), after two years of initial RFA therapy, 95% of the patients had complete eradication of dysplastic changes and 93% had complete eradication of non-dysplastic BE. Similarly, more recent studies have shown comparable eradication rates (70–85%), however, recurrence of BE and associated dysplastic changes are reported between 20% and 24%, with a progression rate to invasive adenocarcinoma of 1.8% (17,18). Longer BE segment on Prague classification, older age, more advanced pretreatment histology (i.e., dysplasia) and non-Caucasian ethnicity were factors associated with a higher risk of disease recurrence (17,18).
Patients with a diagnosis of HGD or intramucosal carcinoma (Figure 1C,D) confirmed by a second expert pathologist should undergo endoscopic therapy (6). EMR is the preferred method for the resection of nodularity and other mucosal abnormalities and is frequently used in patients with HGD to rule out intramucosal adenocarcinoma (13). The use of RFA is recommended after mucosal resection in order to eliminate any remaining dysplastic BE and decrease the risk of recurrent disease (6,19).
The workup for patients with chronic gastroesophageal reflux disease and suspicion of BE has been well established (6). Low-resolution white-light endoscopy or high-resolution/high-definition white light endoscopy are the most common techniques used for the evaluation of these patients. Characteristic mucosal changes under direct visualization, the location of the gastro-esophageal junction, the location of the squamocolumnar junction, and the location of the diaphragmatic indentation are reported using the Prague classification (20). On initial endoscopy for BE, four-quadrant tissue biopsies are obtained at 1-cm intervals (6).
Evaluation of each patient’s co-morbidities and chronic conditions should be performed prior to proceed with any endoscopic therapy in order to decrease the risk of complications and safely prepare them for the procedure.
Pre-operative preparation
The preparation for patients undergoing RFA or EMR is the same as the patients undergoing white-light endoscopy. Recommendations for fasting the night before the procedure are given to the patient in the pre-op visit. Review of medications and diet are also performed and pertinent recommendations are given according to each patient condition.
Equipment preference card
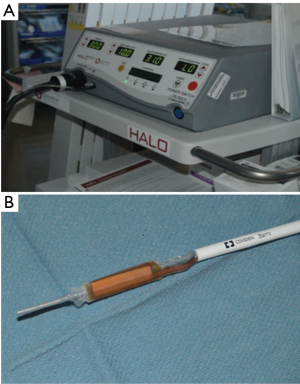
In order to provide RFA to our patients, we prefer to use the HALO360 system (BARRX Medical, Inc., Sunnyvale, California, USA) (Figure 2). This system uses radio frequency energy (J/cm2) in the form of electrical currents and heat to uniformly destroy the esophageal epithelium containing BE (21). The system consists of an energy generator that produces high-power radio frequency energy to the ablation catheter electrode and also provides pressure-regulated air inflation of the sizing balloon and ablation catheter (22). A sizing balloon catheter with different sizes (22, 25, 28, 31 and 34 mm outer diameter) is used to measure the inner diameter of the esophageal area selected for ablation, and the ablation 360-degree balloon catheter containing a 3-cm-long bipolar electrode on its surface delivers the ablative energy (22). There are also the 60-degree, 90-degree and ultra-long focal catheters that fit on the end of the endoscope. The more recent and operator-pleasing advance is the channel catheter which is placed in the working channel of the endoscope. Catheter selection is based on surface area of pathology.
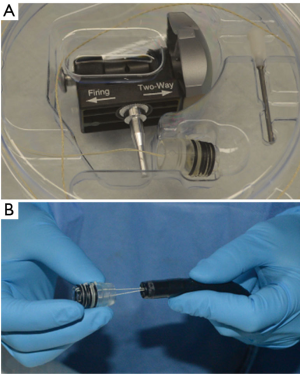
There are two techniques commonly used for mucosal resection, the cap and the band techniques. We preferred to use the band EMR system (DuetteTM, Cook Medical, Limerick, Ireland) for the resection of mucosal abnormalities, HGD and intramucosal adenocarcinoma. This system consists of a transparent cap with six rubber bands that is placed on the tip of the endoscope, a control handle that is assembled at the proximal end of the working channel and is used to deploy the rubber bands, a loading catheter used to connect the trigger cord to the control handle, and a 5-Fr or 7-Fr hexagonal braided polypectomy snare that is used to cut the targeted lesion using blended-current electrocautery (Figure 3) (23,24).
Procedure
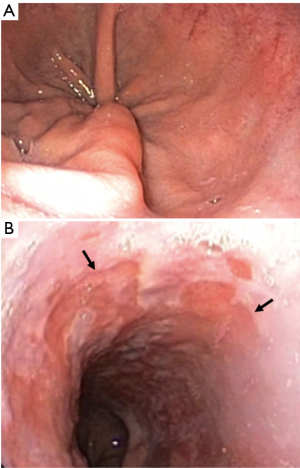
Procedures can be performed with moderate sedation or general anesthesia depending on patient and operator preference. The esophagogastroduodenoscopy is performed without gel lubricant to examine the esophagus and determine the location of the top of the gastric folds (Figure 4A), the location of the proximal extent of BE (Figure 4B), and the presence of any other mucosal abnormalities. All this data is collected and reported according to the C&M Prague classification (20).
RFA
Under direct endoscopic vision, we proceed to determine the area and extension of the esophageal mucosa to be ablated. We then irrigate the esophagus with dilute acetylcysteine solution and introduce the guidewire. Then, over the guidewire we introduce the 22-mm sizing balloon and place it approximately 1 cm above the proximal margin of BE. The sizing balloon is then inflated and complete contact of the balloon with the esophageal wall is assessed. If incomplete contact between the balloon and the esophageal mucosa is evidenced, the sizing balloon is exchanged until complete circumferential contact between these two is achieved, as well as minimal rotational or linear motion within the tubular esophagus. After this, the sizing balloon is withdrawn and the guidewire left in place. Subsequently, the selected ablation balloon is passed over the guidewire and the electrode edge is again placed approximately 1 cm above the proximal extent of BE. At this point, the ablation balloon is inflated and radio frequency energy is delivered using 10 to 12 J/cm2, which takes approximately 3 seconds. Depending on the extension of BE, the balloon is then deflated and advanced to treat all remaining BE until the entire area from 1 cm above the proximal BE margin to the top of the gastric folds is ablated. Afterwards, the ablation balloon is withdrawn and the ablated mucosa is debrided with a cap on the end of the endoscope to facilitate the second ablation cycle, which is performed in the same manner as described above. No debridement is needed after the second round. Finally, the stomach and esophagus are examined and suctioned, and the endoscope is removed (Figure 5).

EMR
Similarly, under direct endoscopic vision the esophagus and stomach are examined and any mucosal abnormalities (nodule, ulcer) or the site where there was biopsy-proven HGD are identified. Then, the endoscope is removed and the EMR system is assembled. After this, the endoscope is re-introduced and the target lesion is identified and suctioned into the transparent cap. Once the cap is filled with esophageal mucosa and the identified lesion is contained with it, the control handle is rotated clockwise and the rubber band is deployed. Subsequently, the suction button is released and the newly created pseudopolyp is visualized. Then, the sheath with the retracted hexagonal snare is introduced and advanced in small increments until is seen in the endoscopic field. Then the snare is pulled out and closed around the target lesion ideally below the rubber band. Once the snare is in an optimal position, electrocautery is used to resect the pseudopolyp. If needed, this procedure can be repeated up to 6 times to ensure complete resection and/or resect multiple lesions. Resected specimens are removed using the suction cap or can be kept in the stomach and be retrieved using a Roth net at the end of the procedure. Finally, after satisfactory resection of the target lesion, careful examination of the base of the mucosectomy is performed looking for any residual BE or abnormal mucosa, exposed blood vessel or any signs of bleeding requiring hemostasis, muscle involvement or esophageal perforation. The stomach is then suctioned out and the endoscope is removed (Figure 6).

Post-operative management
After the procedure, either RFA or EMR, the patients are transferred to the recovery room. Once the patients are in a stable condition, with controlled pain and no signs of complications or hemodynamic instability, they are discharged home with post-operative recommendations and medication instructions.
Medication prescription includes proton pump inhibitors twice daily, pain medication such as acetaminophen oral solution alone or in combination with a moderately potent opioid like codeine 10 mL by mouth every 4 hours as needed. Antacids like magnesium oxide and calcium carbonate can be prescribed to help decrease reflux and post-procedure symptoms. Diet recommendations are also given. Patients should be instructed to eat a soft diet for the next 2–4 days before restarting normal diet, and warning signs for re-consult should be given, including chest pain, fever, abdominal pain, dyspnea, dysphagia and hematemesis, among others.
Post-operative follow-up visit should be scheduled between one and two weeks after the procedure and follow-up endoscopic assessment should be performed between 4–8 weeks after ablation or mucosal resection in order to evaluate the effectiveness of the treatment. Depending on the endoscopic findings, patients can be scheduled for another treatment session or tissue biopsies can be obtained to evaluate pathological response.
Conclusions
A proper assessment and therapeutic approach for patients with dysplastic BE or intramucosal adenocarcinoma is essential to improve outcomes. Early detection of dysplastic changes or EAC will prompt the use of endoscopic therapies such as RFA and EMR. These techniques have shown excellent results in the eradication of dysplasia and intramucosal carcinoma with a significant reduction in the recurrence of the disease and low rate of complications. We believe that the use of endoscopic therapies such as RFA and EMR is effective for the management of this group of patients. We hope this work will familiarize surgeons with these endoscopic techniques which are integral to management of dysplastic BE and early esophageal cancers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chandrasoma PT, Demeester TR. GERD: Reflux to Esophageal Adenocarcinoma. Burlington, Massachusetts: Academic Press, 2010.
- Dresner SM, Griffin SM, Wayman J, et al. Human model of duodenogastro-oesophageal reflux in the development of Barrett's metaplasia. Br J Surg 2003;90:1120-8. [Crossref] [PubMed]
- Chandrasoma P. Controversies of the cardiac mucosa and Barrett's oesophagus. Histopathology 2005;46:361-73. [Crossref] [PubMed]
- Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2006;4:566-72. [Crossref] [PubMed]
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375-83. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol 2016;111:30-50. [Crossref] [PubMed]
- Picardo SL, O'Brien MP, Feighery R, et al. A Barrett's esophagus registry of over 1000 patients from a specialist center highlights greater risk of progression than population-based registries and high risk of low grade dysplasia. Dis Esophagus 2015;28:121-6. [Crossref] [PubMed]
- Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett's esophagus overestimated? Clin Gastroenterol Hepatol 2008;6:159-64. [Crossref] [PubMed]
- Sikkema M, de Jonge PJ, Steyerberg EW, et al. Risk of esophageal adenocarcinoma and mortality in patients with Barrett's esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2010;8:235-44; quiz e32.
- Shaheen NJ, Frantz DJ. When to consider endoscopic ablation therapy for Barrett's esophagus. Curr Opin Gastroenterol 2010;26:361-6. [Crossref] [PubMed]
- Small AJ, Araujo JL, Leggett CL, et al. Radiofrequency Ablation Is Associated With Decreased Neoplastic Progression in Patients With Barrett's Esophagus and Confirmed Low-Grade Dysplasia. Gastroenterology 2015;149:567-76.e3; quiz e13-4.
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Wani S, Abrams J, Edmundowicz SA, et al. Endoscopic mucosal resection results in change of histologic diagnosis in Barrett's esophagus patients with visible and flat neoplasia: a multicenter cohort study. Dig Dis Sci 2013;58:1703-9. [Crossref] [PubMed]
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209-17. [Crossref] [PubMed]
- Kerkhof M, van Dekken H, Steyerberg EW, et al. Grading of dysplasia in Barrett's oesophagus: substantial interobserver variation between general and gastrointestinal pathologists. Histopathology 2007;50:920-7. [Crossref] [PubMed]
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology 2011;141:460-8. [Crossref] [PubMed]
- Guthikonda A, Cotton CC, Madanick RD, et al. Clinical Outcomes Following Recurrence of Intestinal Metaplasia After Successful Treatment of Barrett's Esophagus With Radiofrequency Ablation. Am J Gastroenterol 2017;112:87-94. [Crossref] [PubMed]
- Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett's esophagus. Clin Gastroenterol Hepatol 2014;12:1840-7. [Crossref] [PubMed]
- Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200-6. [Crossref] [PubMed]
- Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392-9. [Crossref] [PubMed]
- Ganz RA, Utley DS, Stern RA, et al. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc 2004;60:1002-10. [Crossref] [PubMed]
- Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc 2007;65:185-95. [Crossref] [PubMed]
- Ge PS, Muthusamy VR. Endoscopic Mucosal Resection for Barrett's Esophagus. J Laparoendosc Adv Surg Tech A 2017;27:404-11. [Crossref] [PubMed]
- Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett's neoplasia. Gastrointest Endosc 2011;74:35-43. [Crossref] [PubMed]
- Muñoz-Largacha JA, Litle VR. Radiofrequency ablation in patients with Barrett’s esophagus. Asvide 2017;4:392. Available online: http://www.asvide.com/articles/1706
- Muñoz-Largacha JA, Litle VR. Endoscopic mucosal resection in patients with Barrett’s esophagus and high-grade dysplasia. Asvide 2017;4:393. Available online: http://www.asvide.com/articles/1707
Cite this article as: Muñoz-Largacha JA, Litle VR. Endoscopic mucosal ablation and resection of Barrett’s esophagus and related diseases. J Vis Surg 2017;3:128.


