Robotic distal pancreatectomy and splenectomy: rationale and technical considerations
Introduction
Minimally invasive distal pancreatectomy has become the most commonly performed technique for distal pancreatectomy in the United States (1). A majority of surgeons had utilized laparoscopic techniques for minimally invasive distal pancreatectomy prior to the advent of modern robotic surgical systems. In contrast to traditional laparoscopy, robotic distal pancreatectomy has been shown to be feasible in performing both standard and more complex resections with greater technical demands (2-4).
To date, there is no standardized approach to minimally invasive distal pancreatectomy to guide surgeons in selecting the most appropriate technique for an individual patient. Cost considerations and surgeon-specific experience or competency level are oftentimes used as the main determinants for performing a specific technique (1,5). With increased availability and a potentially shorter learning curve, robotic distal pancreatectomy may be a useful modality in increasing the successful adoption and application of minimally invasive distal pancreatectomy. The purpose of this report is to describe the rationale and technical approach for the implementation of robotic distal pancreatectomy.
Rationale
Robotic surgical systems provide more instrument range of motion and control compared to traditional laparoscopic instruments. Hand movement in standard laparoscopy leads to exponentially increased instrument movement which makes dissection around sensitive structures challenging. In contrast, robotic surgical systems allow manipulation of the hand to instrument movement ratio, which allows for safe dissection of delicate structures which otherwise require high psychomotor ability. In the situation of a standard distal pancreatectomy, there is limited need to manipulate the hand to instrument movement ratio and does not require significant instrument articulation. Standard port placement and in-line laparoscopic instruments, such as a Maryland dissector and right-angle dissector, are generally adequate for dissection of the splenic vein and artery or other structures in a standard distal pancreatectomy with total splenectomy. In contrast, the use of articulating instruments and manipulating the hand to instrument movement ratio may change the ability to complete a minimally invasive distal pancreatectomy without open conversion in those patients with significant peripancreatic fibrosis, enlarged tumors, or other challenging anatomy. Table 1 provides a relative comparison of traditional laparoscopy and robotic techniques for distal pancreatectomy procedures.
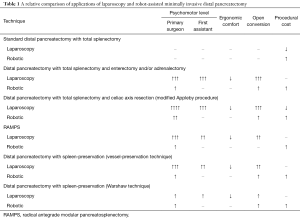
Full table
For patients with locally advanced pancreatic tumors or those warranting a more thorough lymphadenectomy [i.e., radical antegrade modular pancreatosplenectomy (RAMPS)] the use of the robotic system has particular appeal (6). In locally advanced pancreatic body and tail tumors the use of the robotic systems can aid the surgeon in performing en bloc resections of the involved structures such as the duodenum or adrenal gland. Additionally, the robotic system is decidedly more straightforward for the surgeon to perform hand-sewn anastomoses should they be necessary in the case of a bowel anastomosis or oversewing of vessels. The full wrist articulation mimicking the surgeon’s hand can make performing these anastomoses more straightforward, particularly in the case of a surgeon less comfortable with advanced intracorporal suturing skills.
Further, robotic surgical systems are advantageous in RAMPS procedures where the gastroduodenal and infra-pancreatic lymph node basins must be resected to complete the N1 dissection. Clearance of nodal tissue along the right gastroepiploic vein, gastroduodenal artery, and common hepatic artery is believed to be a critical component for the survival advantage noted in RAMPS (6). Although a pure laparoscopic approach may be feasible, many pancreatic surgeons are unlikely to feel comfortable with this dissection given the limited dexterity of current laparoscopic instruments. In minimally invasive RAMPS, careful dissection and mobilization of perivascular lymphatic tissue is greatly facilitated using fully articulating instruments which can also be adjusted to decrease the hand to instrument movement (4).
A final technical modification of the distal pancreatectomy which can be facilitated using the robotic system is spleen-preservation (2,3,7). In spleen preserving techniques where pancreatic branches from the splenic vein and artery are individually ligated and sutured (splenic vessel preservation), robotic surgical systems increase the likelihood of successful splenic preservation compared to traditional laparoscopy (2,3). This effect can be explained by the impact of the robotic instrument articulation providing greater needle dexterity which is critical in ligating small venous or arterial branches along the relatively thin-walled splenic vein. Given the number of sutures required, surgeon comfort also becomes a greater consideration during these types of technically demanding procedures and the improved ergonomics seen with robotic surgical systems can help prevent surgeon discomfort and fatigue throughout the procedure. In comparison, the Warshaw technique (non-splenic vessel preserving) where the splenic vein and artery are divided, the ability to carefully dissect the splenic vein tributaries seen in the diffuse splenic vein anatomy is challenging in pure laparoscopy. Robotic instrumentation with articulation and modification of the hand to instrument movement ratio appears to aid in minimizing blood loss and completing the procedure with a minimally invasive approach.
Considerations
Robotic surgical systems require institutional credentialing prior to use (8). Furthermore, mentorship to develop competency in robotic instrumentation is critical to avoid life-threatening injuries which can be seen with any surgical instrument (8). Although robotic surgical systems are certainly more generalizable to the traditional surgeon compared to laparoscopic techniques, training in safe trochar and robot-specific instrument use must be obtained prior to implementing the technology in clinical practice.
With respect to robotic distal pancreatectomy, trochar placement is similar to those used in laparoscopic distal pancreatectomy. Depending on the robotic surgical system used and preferred instrumentation, the trochars are a combination of either 5, 8, or 12 mm in diameter. The patient should be deemed a safe candidate for pneumoperitoneum and if intraperitoneal adhesions exist then trochar placement may need to be staged with adhesiolysis performed until all trochars can be placed under direct visualization.
Most minimally invasive distal pancreatectomy procedures utilize endoscopic stapling devices to transect the pancreatic parenchyma. Both robotic stapling devices, depending on the surgical system used, and laparoscopic stapling devices can be used. Parenchymal suturing at the transection margin can be performed depending on surgeon preference and does increase the degree of technical challenge encountered compared to open techniques. If a RAMPS procedure is performed, additional trochars are used to aid in performing the hepatoduodenal ligament and infra-pancreatic lymph node dissections. The assistant port in RAMPS procedures is of greater importance to retract or hold structures during the dissection. In the setting of a locally advanced tumor requiring duodenal resection, table manipulation may be needed during the procedure while mobilizing the ligament of Treitz. Although commercially available operative tables are available which coordinate table movement with the robotic system, if not available the robotic system will need to be undocked from the patient to manipulate the operative table during this portion of the procedure.
Technique
The peritoneal cavity can be entered in a variety of methods including traditional laparoscopic techniques or a robot-assisted method. Utilizing the robotic camera with an optical view trochar in the left aspect of the epigastrium is a cost-effective method we utilize to avoid use of laparoscopic equipment. Additional trochars are then placed in the right anterior axillary line, right para-median, supraumbilical, left para-median, and left anterior axillary line. The size of the trochars depends on the robotic device utilized. Examples of port placement are demonstrated for the Intuitive Da Vinci Si and Xi systems for distal pancreatectomy in Figures 1,2. The potential use of smaller trochars such as robotic 5 mm trochars has the advantage of a potentially lower risk for incisional hernia, although the instruments at this time are more limited in the existing robotic systems and not ideal for robotic distal pancreatectomy.
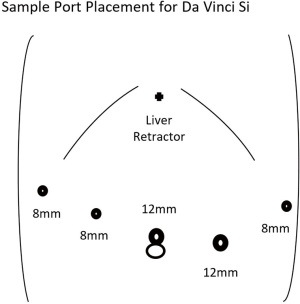
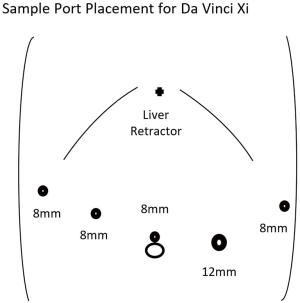
After placement of the robotic trochars, the epigastric trochar is removed and the camera repositioned to the supraumbilical trochar site. A liver retractor such as the Nathanson retractor is placed through the epigastric port site. The robotic surgical system is then docked from either above the head or obliquely depending on the surgical system used. The first assistant is positioned on the patient’s left side and will utilize the left para-median trochar for suctioning and potentially stapled transection of the pancreas. The surgeon at this point moves to the robotic console after ensuring correct placement of the desired instruments. An example of an instrument orientation would be an atraumatic grasping device in the right anterior axillary and left anterior axillary trochars with an ultrasonic dissector or bipolar dissector in the right para-median trochar.
The operation proceeds similar to previous descriptions of distal pancreatectomy depending on the extent of lymphadenectomy or performance of splenic preservation. Figure 3 demonstrates the standard technique for a robot-assisted distal pancreatectomy with splenectomy. For a standard distal pancreatomy with total splenectomy, the gastrosplenic ligament and short gastric vessels are serially divided using the dissecting device up to the level of the left phrenoesophageal ligament. The stomach is grasped and retracted lateral and caudal using the right anterior axillary grasping device while the left anterior axillary grasping device retracts the greater omentum caudal. The right anterior axillary grasping device serially regrasps the posterior aspect of the stomach and rotates the stomach counter-clockwise to better expose the gastric fundus and cardia while dividing the gastrosplenic ligament. The superomedial aspect of the splenodiaphragmatic ligament can be divided at this point as well given the excellent exposure. The liver retractor is re-positioned to retract the stomach and liver anteriorly. Similarly a Penrose drain can be placed to similarly retract the stomach anteriorly.

The gastrocolic ligament is divided in conjunction with the gastrosplenic ligament up to the level of the right gastroepiploic vein depending on the extent of pancreatectomy and lymphadenectomy desired. If a distal pancreatectomy at the level of the superior mesenteric vein is necessary then the right gastroepiploic vein is followed distally to the junction with the superior mesenteric vein while retracting the stomach anteriorly with the left anterior axillary grasping device. The peritoneum overlying the superior mesenteric vein and caudal aspect of the pancreatic neck or body is divided using an electrosurgical device or dissector. The peritoneum along the caudal aspect of the pancreatic body and tail is similarly divided to allow for caudal retraction of the colon and transverse mesocolon to prevent an iatrogenic mesocolic defect.
A retro-pancreatic tunnel is created using blunt dissection with the right and left anterior axillary grasping at the level of the superior mesenteric vein. The dissection ends at the cephalad aspect of the pancreas beyond the level of the splenic vein. The dissection proceeds anteriorly at the cephalad aspect of the pancreas to isolate the splenic artery. The splenic artery should be followed proximally to the celiac trunk and all lymphatic tissue dissected from the splenic artery and celiac trunk to be included with the specimen. A laparoscopic or robotic ultrasound probe should be routinely employed to evaluate the pancreatic parenchyma, identify the pancreatic lesion, and main pancreatic duct. The ultrasound exam is additionally used to guide the level of pancreatic parenchyma transection ensuring an adequate margin is achieved.
Except in the case of splenic vessel preservation, the splenic artery is divided at the level of the celiac trunk or distally to preserve the dorsal pancreatic artery. The splenic artery can be divided using either surgical clips or a surgical vascular stapler load. The splenic vein is then bluntly dissected from the pancreatic parenchyma circumferentially on the posterior aspect of the pancreatic body at the level of the planned parenchymal transection. The splenic vein is divided using either surgical clips or a surgical vascular stapler load. The pancreatic parenchyma can be divided at this step using a variety of transection techniques including a surgical stapling device, electrosurgical dissector, ultrasonic dissector, or sharp transection. If desired the pancreatic transection stump and main pancreatic duct can be over sewn using robotic needle drivers placed through the left anterior axillary trochar.
The pancreatic body and tail are then elevated anteriorly using the right anterior axillary trochar while the transverse colon is retracted caudal. The splenocolic ligament is divided using either a monopolar or a surgical dissecting device to mobilize the splenic flexure of the colon. The splenorenal ligament can be divided at this point with adequate caudal retraction of the transverse colon. The retro-pancreatic lymphatic tissue is then divided using either an ultrasonic or bipolar dissector to complete the retro-pancreatic lymphadenectomy. The remaining splenodiaphragmatic and splenorenal ligaments are divided as well to complete the resection.
There are two predominant methods for specimen removal in minimally invasive distal pancreatectomy with splenectomy. The specimen can be left intact or the distal pancreas can be divided from the spleen and the specimens removed separately. There has been no evidence suggesting a benefit of maintain the specimen intact at the time of removal assuming the lesion is not violated by performing this maneuver. The most commonly utilized extraction site for the specimen is the supra-umbilical trochar site which requires replacement of the robotic camera to the right para-median or left para-median trochar depending on the surgical system utilized. Prior to removal of the specimens a surgical drain can be placed through the left anterior axillary trochar site with removal of the trochar. The specimens are placed within a protective bag to avoid trochar site seeding or contamination. The extraction trochar often requires enlargement for specimen removal. Trochar fascial defects can be closed using either a transfascial or anterior approach.
Conclusions
Robotic distal pancreatectomy is a valuable technique for performing minimally invasive distal pancreatectomy. The increased dexterity afforded by the robotic surgical systems can aid the surgeon, particularly during lymph node dissections such as those in a RAMPS procedure or vascular dissection such as spleen-preserving techniques. Further investigations which will attempt to expand the body of evidence on the role of robotic distal pancreatectomy may be important to clarifying how to best implement the technology.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rosok BI, de Rooij T, van Hilst J, et al. Minimally invasive distal pancreatectomy. HPB (Oxford) 2017;19:205-14. [Crossref] [PubMed]
- Chen S, Zhan Q, Chen JZ, et al. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc 2015;29:3507-18. [Crossref] [PubMed]
- Eckhardt S, Schicker C, Maurer E, et al. Robotic-Assisted Approach Improves Vessel Preservation in Spleen-Preserving Distal Pancreatectomy. Dig Surg 2016;33:406-13. [Crossref] [PubMed]
- Lee SH, Kang CM, Hwang HK, et al. Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc 2014;28:2848-55. [Crossref] [PubMed]
- Magge D, Gooding W, Choudry H, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg 2013;148:525-31. [Crossref] [PubMed]
- Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery 2003;133:521-7. [Crossref] [PubMed]
- Lee LS, Hwang HK, Kang CM, et al. Minimally Invasive Approach for Spleen-Preserving Distal Pancreatectomy: a Comparative Analysis of Postoperative Complication Between Splenic Vessel Conserving and Warshaw's Technique. J Gastrointest Surg 2016;20:1464-70. [Crossref] [PubMed]
- Melvin WS. Robots in surgery: advanced gastrointestinal applications and credentialing. J Gastrointest Surg 2003;7:481-3. [Crossref] [PubMed]
- Royall NA, Walsh RM. Technique for robotic-assisted distal pancreatectomy and splenectomy. Asvide 2017;4:405. Available online: http://www.asvide.com/articles/1719
Cite this article as: Royall NA, Walsh RM. Robotic distal pancreatectomy and splenectomy: rationale and technical considerations. J Vis Surg 2017;3:135.


