Clinical value of endoscopic ultrasonography for esophageal leiomyoma in elder patients
Introduction
Leiomyoma makes up about 1.2% of all esophageal tumors, nowadays, the incidence of this disease is increasing, however, the etiology and pathogenesis of it still remain unknown. At present, there is no criteria in diagnosis and treatment for this disease, and there are few clinical researches about it.
The majority of patients with esophageal leiomyoma remain asymptomatic, and a very few patients go with epigastric discomfort, dysphagia or other symptoms (1-4), which were not specific for the disease. Most cases of the esophageal lesions were found by accident when patients received upper conventional endoscopy for other diseases. But it is still very hard for conventional endoscopy to make a distinction between esophageal leiomyoma and other submucosal lesions or external pressure neoplasms, for it cannot acquire specific information of a lesion’s size, origin, and the relationship with nearby organs, potentially causing diagnostic confusion (2).
Endoscopic ultrasonography (EUS) can clearly reveal the five-layered structure of the esophageal wall, and thereby confirm the nature, size, number, and origin of the lesions (3,5), and it is now an effective method of the diagnosis and treatment to esophageal leiomyomas.
With the development and clinical application of EUS, the incidence of patients with esophageal leiomyomas is increasing, but there are few researches related were reported, especially in elder ones, whose diagnosis, differential diagnosis and treatment has its own particularity, so the clinical value of EUS in esophageal leiomyomas is worth further study.
The purpose of this paper is to describe the EUS features, clinical features, auxiliary examinations features, treatment outcomes and follow-up results in patients with esophageal leiomyoma. Then concluded the clinical features of patients with esophageal leiomyoma, particularly in elder ones, investigating the diagnosis, differential diagnosis and treatment making value of EUS in these patients. In addition, a periodically follow-up with endoscopy was made for a few patients without resection or after resection to analyse the progression of esophageal leiomyoma in elder patients.
Methods
Patients
The patients included in the study were inpatients or outpatients seen at the First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, China), from June 2005 to June 2015.
We first screened all the patients who underwent EUS during this period for suspected esophageal submucosal protuberant lesions, as revealed by conventional endoscopy, and then included 2,134 patients (1,114 males and 1,020 females) who had been diagnosed as esophageal leiomyoma by EUS for further analysis.
The age of all the patients ranged from 15 to 85 years (mean age: 53±10.6 years), of which there are 249 cases (accounted for 11.7%) elder patients (65 years and older), and 1,885 cases (accounting for 88.3%) non-elder patients (under the age of 65 years). Among elder patients, there were 141 men and 108 women, their mean age was 70±4.0 years, ranging from 65 to 85 years. Among non-elder patients, there were 973 men and 912 women, their mean age was 50±8.9 years. There was no significant difference in gender ratio between elder and non-elder patients. The study was approved by the Hospital Ethical Committee, and all the patients agreed to participate in the study.
EUS examinations
The preparations for EUS were similar to those for conventional endoscopy. EUS instruments included a double-cavity electronic endoscopy (GIF-2T-240) with a miniature ultrasonic probes (MUP) (UM-DP12-25R), whose frequency was 12–15 MHz (all equipment were made by Olympus Medical System, Tokyo, Japan). All operations were conducted by professional endoscopic operation personnels. According to the information shown by the conventional endoscopy examination, we chose different frequency microprobes and examination methods (water-balloon method, water-soak method or water-pour method) to scan the lesion (6).
The diagnosis of esophageal leiomyoma by EUS was based on two aspects: (I) as observed on the endoscopic image, a submucosal protruding lesion with an intact mucosal surface and a normal color was seen, the quality of it was medium hardness, and the shape of it was oval or like a peanut; (II) as observed on the ultrasound, a low homogeneous echo tissue structure with clear margins was seen, most esophageal leiomyoma lesions were located within the mucosal layer, the submucosa layer, or muscularis propria, and together with normal surrounding structures. Then a preliminary diagnosis was made according to the features on EUS mentioned above. Observe and record the tolerance and operation-related complications of patients who underwent EUS.
Therapeutic indications
EUS can confirm the nature, size, number, and origin of the lesions (6,7), according to these informations, we choose a follow-up examination, endoscopic resection or surgical excision for further study. If the lesion originates from the mucosal layer or submucosa layer, we give preference to endoscopic resection, such as high-frequency electric snare resection. Some esophageal leiomyomas originating from the muscularis propria may also be appropriate for endoscopic treatment, such as submucosal tunneling endoscopic resection (STER), endoscopic submucosal dissection (ESD) (8,9). If a lesion is difficult for endoscopic therapy, or the patient has obvious symptoms and require for treatment, or the endoscopic therapy was failed for the patient, surgery is preferred.
Follow-up
A periodically follow-up with EUS was made for a few patients without resection or after resection to study the change of lesions and the recurrence after treatment, then conclude the therapeutic indications and progression of esophageal leiomyoma in elder and non-elder patients.
Data analysis
We use SPSS 17.0 software for Windows (SPSS, Chicago, IL, USA) to carry on statistical analysis. Statistically significant was considered as P<0.05 (two-tailed test).
Results
EUS examinations
EUS examinations were well tolerated in elder cases, without serious complications, such as perforation, bleeding, or cardiopulmonary event (n=249).
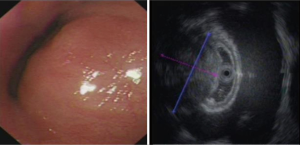
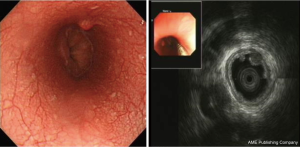
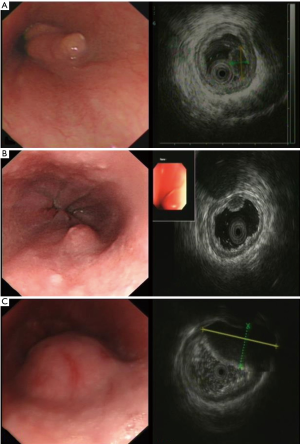
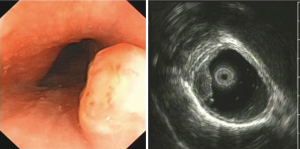
A total of 3.6% elder esophageal leiomyoma patients had more than one lesions. Of all elder esophageal leiomyoma cases studied, 36.4%, 32.6%, and 31.0% lesions were located within the upper, middle, and lower third of the esophagus, respectively. Most cases originated from the mucous layer (60.9%) (Figure 1A), while others originated from the submucosa (15.1%) (Figure 1B) or the muscularis propria (24%) (Figure 1C). The diameters of tumor in elder patients ranged from 5 to 40 mm, with a mean of 7.2±5.97 mm. There was no significant statistical difference in number, locations, origin and size between elder and non-elder patients (P>0.05 in every comparison) (see Table 1).

Full table
Clinical symptoms and underlying disease
Some of the elder esophageal leiomyoma patients were asymptomatic or had mild nonspecific epigastric discomfort, which were not specific for the disease. Most of the lesions were found by accident when patients received epigastric endoscopy for other diseases. Among 249 elder esophageal leiomyoma patients, there were 4 complained of swallowing discomfort, 2 complained of hiccup, 1 complained of dysphagia, only accounting for 2.8% (7/249) in all elder patients. Among non-elder patients, there were 3.1% (58/1885) complained of symptoms related esophageal leiomyoma. There was no significant statistical difference for symptoms related esophageal leiomyoma between elder or non-elder patients (P>0.05).
Two hundred and forty-nine elder patients with esophageal leiomyoma, 33 received endoscopic resection or surgical excision, and 48.5% (16/33) of them accompanied with hypertension, diabetes mellitus, coronary heart disease, chronic bronchitis and chronic obstructive pulmonary disease (COPD). And at the same time, 25.9% (124/479) non-elder patients went with hypertension, diabetes mellitus, chronic bronchitis and gout, which was reported less than elder patients group (P=0.005). No positive physical findings were noted in esophageal leiomyoma patients.
Serological features
Thirty-three elder patients with esophageal leiomyoma received endoscopic resection or surgical excision, and 63.6% (21/33) patients were hospitalized and underwent serological examination, 2 of them had higher carcinoembryonic antigen (CEA) level, 1 had higher carbohydrate antigen (CA) 125 level, the rest 85.7% (18/21) patients had normal blood routine, serum alpha-fetoprotein (AFP), CEA, CA125, CA199 and C-reactive protein (CRP) levels. In non-elder patients, 44.3% (212/479) patients were hospitalized and underwent serological examination, 3 of them had higher CEA level, 6 of them had lower white blood cell (WBC) count and hemoglobin, the rest 95.8% (203/212) patients had normal blood routine, serum AFP, CEA, CA125, CA199 and CRP levels. Compared with non-elder patients, the serological examination indexes had more positive changes in elder patients group, and there was significant statistical difference between them (P=0.047).
Computed tomography (CT) examination features
A total of 63.6% (21/33) elder patients with esophageal leiomyoma in hospital underwent chest CT examination. Chest CT examination showed non-circumferential focal thickening and mildly enhancing of the esophageal wall images in 38% (8/21) patients, 2 of which went with calcification, and the primary diagnosis of these lesions were esophageal wall-thickened or occupied lesions. The rest 62% (13/21) patients showed negative findings on CT scanning. One hundred and twenty-six non-elder patients in hospital underwent chest CT examination. Chest CT examination showed non-circumferential focal thickening images in 39 patients and the primary diagnosis of these lesions were esophageal wall-thickened or occupied lesions. The rest 69.0% (87/126) patients showed negative findings on CT scanning. There was no significant statistical difference in spiral CT images between elder and non-elder patients (P>0.05).
Therapeutic effects and complications
Among 13.3% (33/249) elder patients with esophageal leiomyoma who underwent treatment, there were 30 patients received endoscopic resection and 3 patients received surgical excision according to the origin of the lesion. One patient with COPD cannot tolerate anesthesia treatment and gave up endoscopic resection, so the success rate of the endoscopic resection was 96.7% (29/30). Three patients received surgical excision successfully. The size of resected tumor ranged from 2.2 to 40 mm (mean size 8.6±8.7 mm), 12.1% (4/33) of lesions were bigger than 10 mm. And among them, 27, 3, and 3 lesions were originated from the mucous layer, submucosa and muscularis propria of the esophagus, respectively. A total of 6.1% (2/33) patients had postoperative infection, no other treatment-related complications occurred, such as bleeding or perforation.
Among 25.4% (479/1885) non-elder patients with esophageal leiomyoma who underwent treatment, there were 454 patients received endoscopic resection and 25 patients received surgical excision according to the origin and size of the lesion. Five patients gave up endoscopic resection because of bleeding or other reasons and changed to receive surgical excision, so the success rate of the endoscopic resection was 98.9% (449/454). Twenty-five patients received surgical excision successfully. The size of resected tumor ranged from 1.6 to 58 mm (mean size 7.1±6.6 mm), 16.7% (80/479) of lesions were bigger than 10 mm. And among them, 344, 77, and 58 lesions were originated from the mucous layer, submucosa and muscularis propria of the esophagus, respectively. In all patients received endoscopic resection, 5 patients had postoperative infection and 1 patient had excessive bleeding, and the incidence of complications was 1.3%. No treatment-related complications occurred in patients received surgical excision.
Compared with non-elder patients, fewer elder patients chose endoscopic or surgical therapy (P<0.001). There was no significant statistical difference in the size of resected tumor specimens between elder and non-elder patients (P>0.05). The incidence of complications was significantly higher in elder patients, especially postoperative infection (P=0.039), and the number of inpatients of elder was higher ratio than non-elder, P=0.031 (Table 2).

Full table
Histological diagnosis
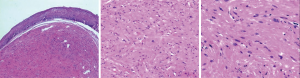
Histological diagnosis of esophageal leiomyoma was on the basis of proliferation of spindle cells arranged in beam or weave pattern, and immunohistochemical staining showed positive SMA and negative CD117 staining ( Figure 2). Postoperative pathological results of 81.8% (27/33) patients in elder group and 86.2% (413/479) patients in non-elder group were completely consistent with the preoperative diagnosis of EUS.
We misdiagnosed 6 (18.2%) cases in elder patients and 66 (13.8%) cases in non-elder patients. Among non-elder patients, we misdiagnosed 59 cases in 454 patients who received endoscopic resection, and 7 cases in 25 patients who received surgical excision (Table 3).

Full table
The misdiagnosis rate of malignant tumors was significant higher in elder group (33.3%) than in non-elder group (6.1%) (P=0.021), and most misdiagnosis cases in non-elder group were cystic gland retention and inflammation.
Follow-up informations
Seventy-one (28.5%) elder patients without treatment chose to be followed-up (mean: 532 days, range: 1–66 months) by endoscopy or EUS examination. Among them, tumors grew quickly in 4 cases (4/71, 5.6%), and 1, 2, and 1 lesions were diagnosed as squamous carcinoma, stromal tumor, external pressure neoplasm, respectively. The follow-up examinations in the rest patients (94.4%) showed that the size grew about 0.03 mm per year, almost without change in size.
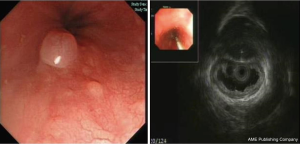
A total of 535 (28.4%) non-elder patients without treatment chose to be followed-up (mean: 476 days, range: 1–56 months), and the examinations showed no changes in tumor size, just the same as elder group (Figure 7).
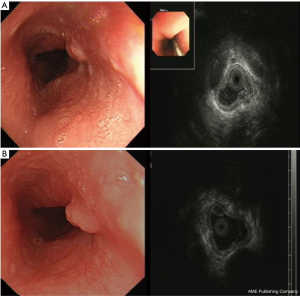
For the elder patients who received treatment, no recurrence was observed during the follow-up period (mean: 15 months, range: 6–36 months). Resected lesions by endoscopic therapy restored to normal mucosa after about 2 months (Figure 8).
Discussion
EUS features of elder patients
Our study showed that both elder and non-elder patients have good tolerance for EUS examination, even elder patients accompanied with more underlying diseases, EUS examination can be processed successfully. And elder patients with esophageal leiomyoma accounted for 11.7% in all cases, patients can be male or female, and male patients were a little more than female patients. In most cases, there was only 1 lesion (96.4%) and the lesion size was less than 10 mm. The lesions can occur in any part of the esophagus, but the majority was located in the middle and upper thirds of the esophagus. Most lesions originated from the mucous layer (60.9%), while the rest of the lesions originated from the submucosa layer or the muscularis propria. All features of the lesions in elder group had no significant difference with non-elder group.
Clinical features of elder patients
Most lesions in elder patients were small with a benign course, patients usually remained asymptomatic. Even larger lesions had little symptoms such as swallowing discomfort or choke, and there was no significant difference with non-elder patients. No positive physical findings were noted in all patients. Most serological features of elder inpatients were within normal limits, when compared with non-elder patients, serologic abnormalities such as tumor markers were more common. However, in 3 elder patients with abnormal serum tumor markers in our study, 2 of them were cancer patients (one was lung cancer infiltration of the esophagus misdiagnosed as esophageal leiomyoma; the other had colon cancer and esophageal leiomyoma at the same time), which suggested that serologic abnormalities may have nothing to do with esophageal leiomyoma, and were associated with complex underlying conditions of elder patients. The results prompt us that once elder patients were diagnosed as esophageal leiomyoma by EUS, and had abnormal serum tumor markers at the same time, we should not only think about the possibility of esophageal leiomyoma, but also be vigilant about the superficial esophageal carcinoma or metastatic carcinoma. The rate of patients accompanied with hypertension, diabetes mellitus, chronic bronchitis and other diseases were significantly higher in elder ones, and the condition is more complex.
The diagnosis value of conventional endoscopy and spiral CT
Conventional endoscopy can reveal the lesion and distinguish it from polyp, cancer and other diseases, but it is unable to acquire exact information including the size, origin or the relationship with the surrounding organs of a lesion, and it is also hard for conventional endoscopy to differentiate between esophageal leiomyoma and external pressure neoplasms or other submucosal lesions.
Spiral CT might find a thickening of the esophageal wall when a lesion was larger than 10 mm, but it went with low sensitivity in showing an esophageal lesion less than 10 mm, besides, it is also unable to confirm the nature, origin, or surface of a lesion (5).
The diagnosis value of EUS
Except for showing the esophageal lesions, EUS could also assess the nature, size, number, location, origin of lesions, and their relationship with the surrounding organs. With these information, EUS was able to make a distinction between esophageal leiomyoma and other submucosal lesions or external pressure neoplasms, such as inflammatory nodule, hemangioma, intravenous nodule, lipoma and cyst. In our study, preoperative diagnostic accuracy of EUS for esophageal leiomyoma is 81.8% in elder patients and 86.2% in non-elder patients, which is thought to be the best diagnosis method at present, and is obviously superior to conventional endoscopy and CT.
However, we misdiagnosed 18.2% elder patients and 13.8% non-elder patients, there was still a gap between EUS diagnosis and pathological diagnosis. Most misdiagnosis cases were cancer or other diseases in elder patients and cystic gland retention or inflammation in non-elder patients, the misdiagnosis rate of malignant tumors was significant higher in elder group than in non-elder group (33.3% vs. 6.1%, P=0.021). In elder patients who were misdiagnosed, they may have abnormal serum tumor markers at the same time, which reminded us to be vigilant about the superficial esophageal carcinoma or metastatic carcinoma, and even with small lesions, we also advise these patients to have a fine needle aspiration (FNA) or an excision.
Therapeutic indications in elder patients
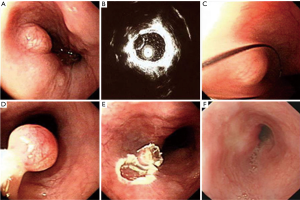
Our study showed that EUS is a scientific and reasonable technique for making treatment decisions of the patients with esophageal leiomyoma. If the lesion originates from the mucous layer or submucosa layer, we give preference to endoscopic therapy, such as endoscopic mucosal resection (EMR) (Figure 9), and some esophageal leiomyomas originating from the muscularis propria can also select the endoscopic therapy, such as ESD (Figure 10) or STER (Figure 11). And in general, surgery is suitable for lesions originating from the muscularis propria, on account of larger lesions and potential postoperative complications, like massive bleeding and perforation. And in this study, we showed that according to these results of EUS, we can choose scientific, reasonable and personalized methods to manage patients with esophageal leiomyomas, and endoscopic therapies in elder patients were not inferior to that in non-elder patients, in addition, we can obtain specimens after treatment to do pathological examination to find stromal tumor, carcinoid tumor, metastatic tumor and other potentially malignant or malignant lesions in time.
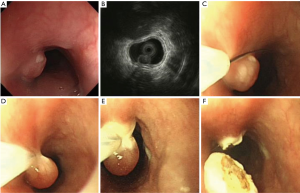
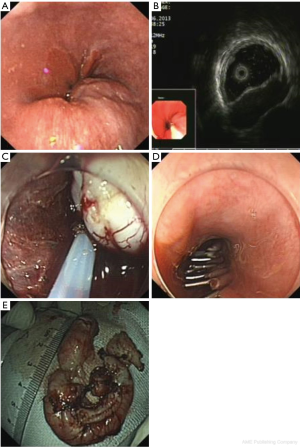
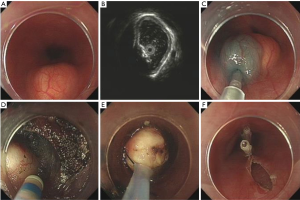
However, compared with non-elder patients, fewer elder patients chose endoscopic or surgical therapy (13.3% vs. 25.4%, P<0.001), which indicated that elder patients tend to choose more conservative methods to deal with their diseases, if the lesion originates from mucous layer or submucosa was small, asymptomatic, stable and the patient has no obvious treatment requirements, they usually chose a follow-up examination or an endoscopic therapy for it is safe and easy.
Once received treatment, there was no difference in the operative successful rates between elder and non-elder patients, but the incidence of complications was significantly higher in elder patients, especially postoperative infection (6.1% vs. 1.3%, P=0.039), and the number of inpatients of elder group was higher than non-elder (63.6% vs. 44.2%, P=0.031), these results suggested that elder patients may suffer more complicated situations after treatment, this was because they may be accompanied with more underlying diseases and have poor tolerance for treatment, so we should have more strict therapeutic indications for elder patients, and the postoperative monitoring should be strengthened for them.
Prognosis and follow-up
The growth of esophageal leiomyoma was slow, and the lesion remained stable during the follow-up period of 3–5 years. Even larger lesions had little local and systemic clinical manifestations. No recurrence was observed after endoscopic or surgical resection during the follow-up.
For those patients without strong desire to be treated, if their lesions from muscularis propria are less than 20 mm, patients can choose to be periodically followed-up, in order to monitor the disease progression and prevent excessive treatment. If the lesions grow larger in a short time, we should pay attention to the possibility of malignant tumors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Hospital Ethical Committee (No. 2016391), and all the patients agreed to participate in the study.
References
- Punpale A, Rangole A, Bhambhani N, et al. Leiomyoma of esophagus. Ann Thorac Cardiovasc Surg 2007;13:78-81. [PubMed]
- Mutrie CJ, Donahue DM, Wain JC, et al. Esophageal leiomyoma: a 40-year experience. Ann Thorac Surg 2005;79:1122-5. [Crossref] [PubMed]
- Asteriou C, Konstantinou D, Lalountas M, et al. Nine years experience in surgical approach of leiomyomatosis of esophagus. World J Surg Oncol 2009;7:102. [Crossref] [PubMed]
- Jiang W, Rice TW, Goldblum JR. Esophageal leiomyoma: experience from a single institution. Dis Esophagus 2013;26:167-74. [Crossref] [PubMed]
- Choi SH, Kim YT, Han KN, et al. Surgical management of the esophageal leiomyoma: lessons from a retrospective review. Dis Esophagus 2011;24:325-9. [Crossref] [PubMed]
- Xu GM, Niu YL, Zou XP, et al. The diagnostic value of transendoscopic miniature ultrasonic probe for esophageal diseases. Endoscopy 1998;30 Suppl 1:A28-32. [PubMed]
- Nomura N, Goto H, Niwa Y, et al. Usefulness of contrast-enhanced EUS in the diagnosis of upper GI tract diseases. Gastrointest Endosc 1999;50:555-60. [Crossref] [PubMed]
- Shen EF, Arnott ID, Plevris J, et al. Endoscopic ultrasonography in the diagnosis and management of suspected upper gastrointestinal submucosal tumours. Br J Surg 2002;89:231-5. [Crossref] [PubMed]
- Gress F, Schmitt C, Savides T, et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest Endosc 2001;53:71-6. [Crossref] [PubMed]
Cite this article as: Jiang T, Yu J, Chen L, Chen H, Shan G, Yang M, Xu G. Clinical value of endoscopic ultrasonography for esophageal leiomyoma in elder patients. J Vis Surg 2017;3:137.