Robotic distal pancreatectomy combined with celiac axis resection
Introduction
Modern management of pancreatic adenocarcinoma requires multimodality therapy to enhance overall survival, but complete surgical resection remains the most important component. Unfortunately, nearly 80% of patients present with unresectable disease, either due to metastasis or—less commonly—locally advanced disease in which the tumor abuts or encases regional vasculature. The National Comprehensive Cancer Network (NCCN) guidelines define locally advanced pancreatic body and tail tumors as those with involvement of the celiac axis with relative sparing the gastroduodenal artery (GDA) (1). Historically considered unresectable, this cohort of T4 tumors were not offered resection due to concerns of increased operative morbidity and mortality in the face of aggressive disease biology. A recent autopsy study however, demonstrated that a subset of patients with locally advanced pancreatic cancer will succumb to local disease without evidence of metastasis (2). Such data provide a rationale for attempting aggressive local surgical resection in carefully selected T4 tumors.
Lyon H. Appleby initially described his now eponymous procedure in 1952 for locally advanced gastric cancer. The procedure consisted of an en bloc gastrectomy, distal pancreatectomy, splenectomy, and celiac axis resection (3). A modified version of this procedure (omitting the gastrectomy) has been adopted for locally advanced pancreatic body and tail cancer, now termed the modified Appleby or distal pancreatectomy with celiac axis resection (DP-CAR). A number of single institutional studies have shown this procedure to be feasible (4-9), however concerns remain regarding the additional morbidity compared to a standard distal pancreatectomy and splenectomy. A recent NSQIP study for example reported that DP-CAR was associated with higher mortality comparted to standard distal pancreatectomy, however the analysis was limited by relatively low numbers of procedures performed at each participating institution and the inclusion of low volume centers and surgeons (10). In contrast, two studies from high volume pancreatic centers recently demonstrated that DP-CAR is safe and feasible if performed by experienced pancreatic surgeons, reporting oncologic outcomes that rival resectable and borderline resectable disease (11,12).
At the University of Pittsburgh, we have attempted to curtail some of the morbidity of pancreatic resections by applying minimally invasive—in particular robotic—platforms to complex gastrointestinal (GI) procedures such as the DP-CAR. Recently, we summarized our 30-case experience and compared 19 open to 11 robotic DP-CARs performed between 2008–2015; morbidity was acceptable in both groups while a decrease in operative time, blood loss, blood transfusion was observed in the robotic cohort (12). Notably, no robotic cases were converted to open DP-CAR and median survival in both groups was nearly 3 years. Based on our experience, we herein highlight our robotic DP-CAR case selection criteria and technique.
Patient selection and preoperative workup
In order to be considered for a DP-CAR at our institution, patients must meet the following criteria: (I) biopsy proven pancreatic body/tail tumor with involvement of any of the branches of the celiac axis, without affecting the trunk itself; (II) the GDA must be present and free of disease; (III) all patients must be eligible for, and have received neoadjuvant therapy in the form of chemotherapy (with or without radiation) and (IV) the patient must have a reasonable performance status.
Patients are imaged preoperatively using a triple phase contrast-enhanced CT scan of the chest, abdomen, and pelvis to delineate vascular anatomy and rule out metastatic disease. Endoscopic ultrasound with fine needle aspiration (FNA) is used for biopsy confirmation of the tumor, which is required for administration of neoadjuvant therapy. A cancer antigen 19-9 (CA19-9) level is also checked and used to monitor response to neoadjuvant therapy. Case discussion at a multidisciplinary conference, involving medical oncology, surgical oncology, radiation oncology, pathology, and radiology, is crucial to proper patient selection.
The patient typically receives one of two neoadjuvant chemotherapy regimens, FOLFIRINOX or gemcitabine-nab paclitaxel. Duration of neoadjuvant therapy is variable but we generally favor a 3–6-month course. Regular interval restaging with physical examination, CA19-9 and CT is performed at 2 monthly intervals. A rise in the CA19-9 during neoadjuvant therapy is a poor prognostic marker, and an indication to switch chemotherapy regimens and delay surgery. In a recent analysis of neoadjuvant therapy for borderline resectable and locally advanced tumors at our institution, no patient with a rising CA19-9 during neoadjuvant therapy was able to achieve an R0 resection (13). Radiation therapy is favored at some institutions in the neoadjuvant setting, however currently its role remains unclear
Surgery is typically undertaken within 4 weeks of completion of neoadjuvant therapy. The decision to pursue robotic versus open surgery is primarily dictated by surgeon preference and level of expertise with the robotic platform. In preparation for surgery, an ERAS protocol may be employed.
Equipment preference card
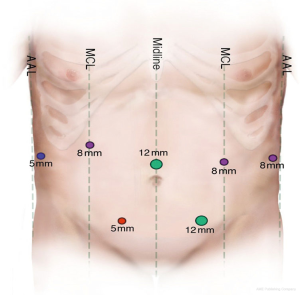
Two laparoscopic 5-mm ports; three robotic 8-mm portal; one 12-mm laparoscopic port for the camera; one 12-mm laparoscopic working port; da Vinci Si Surgical System (Intuitive surgical); 3.0–4.0-mm tissue (purple) Tri-Staple Endo GIA 60-mm cartridge (Covidien, St. Louis, MO, USA) for pancreatic neck transection; 2.0–3.0-mm vascular (gold) Tri-Staple Endo GIA 45-mm cartridge (Covidien, St. Louis, MO, USA) [for common hepatic artery (CHA), splenic vein, left gastric pedicle]; laparoscopic suction; laparoscopic liver retractor; LigaSure (Covidien, St. Louis, MO, USA); 15-mm Endo Catch bag; robotic Doppler ultrasound; 19-mm fluted Blake drain; #1 Vicryl for fascial closure of 12-mm port site and utility extraction site; 4-0 Monocryl for subcuticular closure.
Patient positioning and role of team members
The patient is placed supine on a split leg table to allow the laparoscopic/bedside assistant easy access to all ports. The table is placed in steep Trendelenburg position and the left arm is tucked. All pressure points are padded. Central venous and arterial lines are placed for hemodynamic monitoring. The operating room bed is placed at 45 degrees angle from the anesthesia machine to allow the robot to be docked over the head. Extra-long endotracheal and intravenous (IV) tubing may be needed, since the patient’s head is now further away from the anesthesia cart. The operating team consists of an operating surgeon at the robotic console, a surgical assistant at the bedside, the anesthesiologist, the surgical technician, and the circulating nurse. All members of the operating room staff must be familiar with robotic surgery, particularly patient positioning and robot-specific instrumentation. Additionally, the operative team should be familiar with the processes and procedures needed to safely and efficiently convert a case to laparotomy.
Procedure
Here, we provide a video of the procedure (Figure 1). Laparoscopic and robotic port placement is shown in Figure 2. We begin by performing a diagnostic laparoscopy to evaluate for peritoneal spread. In the absence of metastasis, the robot is docked over the head of the patient. The lesser sac is opened and borders of the pancreas are defined. The short gastric vessels are divided with the LigaSure taking care to preserve the right gastroepiploic vessel. The stomach is then retracted to put the left gastric artery and vein under stretch, thereby exposing the neck and body of the pancreas. We then proceed to the medial dissection. Here, the CHA is traced along the superior border of the pancreas distally to locate the takeoff of the GDA. The CHA is test clamped and ultrasound is used to assess the adequacy of collateral flow within the proper hepatic and right and left hepatic arteries. If there is insufficient triphasic flow in these vessels, the robotic DP-CAR should be aborted and consideration given to perform an open DP-CAR with a jump graft from the aorta to the proper hepatic artery.

In the presence of sufficient flow, the pancreatic neck is encircled and transected using a 3.0–4.0-mm Tri-Staple Endo GIA (Covidien, St. Louis, MO, USA) to the left of the GDA. The splenic vein is dissected at its confluence with the superior mesenteric vein (SMV) and transected with a stapler. We then trace the inferior border of the pancreas laterally, with care to identify and ligate the inferior mesenteric vein using a vascular stapler or the ligaSure. The splenic flexure is lowered and the splenorenal and splenocolic ligaments are divided.
The superior dissection is then performed. The CHA is transected using a 2.0–3.0-mm curved-tip vascular Tri-Staple Endo GIA (Covidien, St. Louis, MO, USA), with care taken to preserve the origin of the GDA. The CHA is followed proximally to the celiac axis, where the left gastric artery and vein are transected using a 2.0–3.0-mm curved-tip vascular stapler. The aorta is exposed superior to the celiac trunk and is traced inferiorly until the celiac trunk is located.
Attention is then turned to the inferior dissection. The superior mesenteric artery (SMA) is identified posterior to the pancreas and dissected proximally to its origin from the aorta. At this level, decussating crural fibers are transected exposing the celiac trunk. Using the robotic hook, all lymphatic and perineural tissue surrounding the aorta and celiac trunk is cleared. Confirmation of the location of the SMA and celiac trunk is aided by the use of the robotic ultrasound. Ultrasound is again used to document adequacy of flow through the proper hepatic artery and the porta hepatis prior to transection. The celiac axis is then transected using a 2.0–3.0-mm curved-tip vascular. A 15-mm Endo Catch bag is used to remove the specimens, which is exteriorized after enlarging the left lower quadrant port site. A 19-mm fluted Blake drain is placed in the resection bed and left to bulb suction
Post-operative management
We employ a pancreas-specific ERAS protocol for DP-CAR and other pancreatic resections. Patients are managed post-operatively on a surgical rather than the ICU. The nasogastric tube is removed upon extubation. Multimodal analgesia is employed utilizing a combination of intrathecal morphine or subcutaneous nerve blocks, ketamine or lidocaine for the initial 48 hours, non-steroidal anti-inflammatory drugs (NSAIDS) and acetaminophen. Narcotics are minimized. Venous thrombophylaxis is started on the evening of the operative day, in addition to aspirin if a concomitant portal vein resection is performed. The patient is allowed clear liquids in the recovery room and diet is advanced as tolerated on postoperative day 1. The Foley catheter is removed the morning after surgery. A drain amylase is checked on the first and third postoperative days and—in the absence of leak (ISGPF definition)—is removed on postoperative day 3 or 4. Intravenous fluid rates are kept to a minimum. In addition to known complications observed after distal pancreatectomy, liver abscess and gastric ischemia are unique complications following DP-CAR. The latter complication is suspected in the presence of delayed gastric emptying in conjunction with a leukocytosis and fever. It is best managed with nil per os (NPO), fluid resuscitation and antibiotics. Occasionally prolonged parenteral nutrition is needed.
Tips, tricks, and pitfalls
Judicious patient selection is critical to reducing the morbidity and mortality of this procedure. We advocate that all patients receive neoadjuvant therapy. While on neoadjuvant therapy, serial CA19-9 levels and CT’s should be used to guide chemo-responsiveness, identify disease progression, and select patients that may benefit from DP-CAR. If preoperative radiation is used, surgery should be ideally performed within 4 weeks of completion of radiation. Familiarity with the anatomic landmarks including the crura, the SMA and the neuro-lymphoid plexus surrounding the celiac trunk is important. Furthermore, familiarity and experience with the robotic platform prior to attempting a robotic DP-CAR is strongly advised; our group only attempted robotic DP-CARs after accumulating sufficient experience with robotic distal pancreatectomy and pancreaticoduodenectomy.
Careful assessment of preoperative imaging is essential. The use of intra-operative ultrasound to confirm the location of the SMA and celiac trunk, and the adequacy of collateral flow in the setting of temporary (test clamp) CHA occlusion cannot be overemphasized. Lastly, attempting this procedure in a center unfamiliar with the post-operative care of pancreatic surgery patients is unsafe, as even in the most experienced hands, more than half of all patients will experience a complication.
Conclusions
The robotic DP-CAR is a safe and technically feasible approach for highly selected locally advanced body and tail pancreatic adenocarcinomas after neoadjuvant therapy. As neoadjuvant therapies and minimally invasive pancreatic surgery techniques evolve, an increasing subset of patients with locally advanced disease will be candidates for this aggressive surgical approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2014;12:1083-93. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [Crossref] [PubMed]
- Appleby LH. The coeliac axis in the expansion of the operation for gastric carcinoma. Cancer 1953;6:704-7. [Crossref] [PubMed]
- Baumgartner JM, Krasinskas A, Daouadi M, et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic adenocarcinoma following neoadjuvant therapy. J Gastrointest Surg 2012;16:1152-9. [Crossref] [PubMed]
- Tanaka E, Hirano S, Tsuchikawa T, et al. Important technical remarks on distal pancreatectomy with en-bloc celiac axis resection for locally advanced pancreatic body cancer (with video). J Hepatobiliary Pancreat Sci 2012;19:141-7. [Crossref] [PubMed]
- Hirano S, Kondo S, Hara T, et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg 2007;246:46-51. [Crossref] [PubMed]
- Okada K, Kawai M, Tani M, et al. Preservation of the left gastric artery on the basis of anatomical features in patients undergoing distal pancreatectomy with celiac axis en-bloc resection (DP-CAR). World J Surg 2014;38:2980-5. [Crossref] [PubMed]
- Wu X, Tao R, Lei R, et al. Distal pancreatectomy combined with celiac axis resection in treatment of carcinoma of the body/tail of the pancreas: a single-center experience. Ann Surg Oncol 2010;17:1359-66. [Crossref] [PubMed]
- Ham H, Kim SG, Kwon HJ, et al. Distal pancreatectomy with celiac axis resection for pancreatic body and tail cancer invading celiac axis. Ann Surg Treat Res 2015;89:167-75. [Crossref] [PubMed]
- Beane JD, House MG, Pitt SC, et al. Distal pancreatectomy with celiac axis resection: what are the added risks? HPB (Oxford) 2015;17:777-84. [Crossref] [PubMed]
- Peters NA, Javed AA, Cameron JL, et al. Modified Appleby Procedure for Pancreatic Adenocarcinoma: Does Improved Neoadjuvant Therapy Warrant Such an Aggressive Approach? Ann Surg Oncol 2016;23:3757-64. [Crossref] [PubMed]
- Ocuin LM, Miller-Ocuin JL, Novak SM, et al. Robotic and open distal pancreatectomy with celiac axis resection for locally advanced pancreatic body tumors: a single institutional assessment of perioperative outcomes and survival. HPB (Oxford) 2016;18:835-42. [Crossref] [PubMed]
- Boone BA, Steve J, Zenati MS, et al. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol 2014;21:4351-8. [Crossref] [PubMed]
- Greer J, Zureikat AH. Robotic distal pancreatectomy with celiac axis resection. Asvide 2017;4:440. Available online: http://www.asvide.com/articles/1757
Cite this article as: Greer J, Zureikat AH. Robotic distal pancreatectomy combined with celiac axis resection. J Vis Surg 2017;3:145.


