Thoracoscopic lobectomy for locally advanced-stage non-small cell lung cancer is a feasible and safe approach: analysis from multi-institutional national database
Introduction
Video-assisted thoracoscopic lobectomy (VATS-L) is a well-established approach for early-stage non-small cell lung cancer (NSCLC) (1-3) and is associated with a shorter length of stay, less post-operative pain, preserved pulmonary function, fewer post-operative complications and better compliance with adjuvant chemotherapy than lobectomy via thoracotomy (4-6). Furthermore, several and authoritative authors demonstrated the efficacy of VATS lobectomy in terms of oncological results and validity of mediastinal intra-operative staging (7-10), but the use of VATS-L for locally advanced-stage NSCLC is not well established. Some preliminary and single-centre retrospective studies have shown that VATS-L is feasible, safe and effective with long-term oncologic outcomes comparable to lobectomy via thoracotomy (11-13).
The objective of this retrospective multi-institutional study was to confirm the safety and feasibility of thoracoscopic lobectomy in locally advanced-stage NSCLC and to compare the peri-operative outcomes with early-stage tumours using a national multi-institutional database, the Italian VATS Group Database.
Methods
Data source
The Italian VATS Group Database is a multicentre, web-based data system for collecting and reporting clinical characteristics, patterns of care, and outcomes data on NSCLC patients treated with a VATS-L. The Italian VATS Group has maintained this prospective database since January 2014. At the time of the latest report, there were more than 54 participating centres (general thoracic surgery units or services, not individual surgeons) and about 4,000 collected cases. Harvested data are maintained by the VATS Group Board and collected on a standardized data form that includes information about patient demographics, medical history, surgical procedures, cancer staging, and outcome. Patients are reviewed and records are updated the first time at 30 days after surgery, then at 180 days. Next update is recorded at 6 months from surgery and every 6 months for the first 2 years of follow-up, and annually thereafter. The Institutional Review Board has provided approval for the data collection, transmission and storage, as well as analyses of the data (No. 81/2014/O/Oss). The current analysis was reviewed and approved for scientific merit and feasibility by the VATS Group Scientific Committee and presented at the annual VATS Group meeting. The VATS Group Database implements rigorous quality assurance and safety procedures to maintain a high level of accuracy and security of data. These include real-time Web-based edit checking, quality assurance reports that are provided by the data managers and on-site audits of a random sample of source documents against the submitted data performed by a Quality Committee. Security features include firewall security, web authentication password protected access, and data encryption transmissions over the internet. To be included in the database, patients must meet the criterion of a VATS-L using a standard approach as it has been defined by VATS Group policy: surgery performed by monitor vision, access incision smaller than 6 cm without rib spreading, one to three additional 1-cm ports, individual dissection of hilar structures with associated lymphadenectomy, use of an endo-bag for specimen extraction.
Patient population and methods
The study population consists of patients who received VATS-L as the primary procedure for locally advanced clinical stage NSCLC as defined by: tumours with dimension >5 cm (cT2b, cT3), cT4 [based on the seventh classification of American Joint Committee on Cancer (AJCC) (14)] and/or tumours that received neoadjuvant chemotherapy at VATS Group participating centres and included in the VATS Group database between 1st January 2014 and 31th May 2017.
Patients with these characteristics were divided into two groups and compared according to the clinical stage: the first group identified as “early-stage group” (group A) comprising clinical stage IA, IB and IIA while the second group, identified as “locally advanced-stage group” (group B), comprising all other patients in clinical stage IIB, IIIA or more. All patients underwent conventional pre-operative examinations, including cardiopulmonary function tests, contrast enhanced thoracic and abdominal computed tomography (CT) scan, brain CT scan and positron emission tomography-CT (PET-CT) scan. In case of mediastinal lymph node CT enlargement or PET-CT scan hyperactivity, endobronchial ultrasound-guided fine-needle aspiration (EBUS-FNA) or mediastinoscopic biopsy was performed before surgery. Restaging was completed with thoracic and abdominal CT-scan, PET-CT scan and/or EBUS-FNA or mediastinoscopy.
To evaluate the safety of VATS-L in locally advanced-stage NSCLC, we compared mortality rate, overall complication rate, frequency and the type of complications. The effectiveness and oncological adequacy of VATS-L was assessed comparing conversion rate, intra-operative data (operative time, estimated blood loss), resection status and number of dissected lymph nodes.
Statistical methods
Statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Standard descriptive statistics have been used to summarize data, with respect to demographic and oncological characteristics. Continuous variables, expressed as mean value ± standard deviation (SD), were compared by unpaired Student’s t-tests; categorical variables were analysed by means of Chi-square tests. A P value below 0.05 was considered as statistically significant.
Results
After exclusions, n=3,720 VATS-L were identified (male: n=2,235, 60.0%, mean age: 67.93 years) among 50 VATS Group affiliated centres. Dividing the study cohort according to the clinical stage, 3,266 (87.8%) patients were included in group A, while 454 (12.2%) patients formed group B. Pre-operative characteristics are depicted in the Table 1. Patients of group B were more frequently male, older, with lower pulmonary reserve and with a tumour localized in the lower lobes.
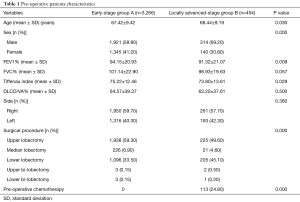
Full table
Surgical approach and type of lymphadenectomy did not differ between the two groups, while total number of dissected lymph nodes and the number of dissected N1 and N2 lymph nodes were statistically different between the two groups (Table 2).
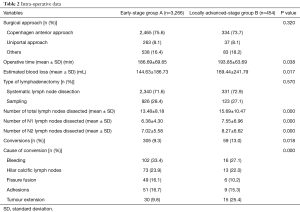
Full table
VATS-L for locally advanced-stage NSCLC was associated with a longer procedure, with a higher estimated blood loss (144.63 vs. 169.44 mL; P=0.017) and an increased incidence of conversion (9.3% vs. 13.0%, P=0.018). The most common causes of thoracotomy in group B were: bleeding (16/59, 27.1%), an unexpected tumour extension precluding a safe thoracoscopic dissection (15/59, 25.4%) and a calcific hilar adenopathy (13/59, 22.0%).
Patients of group B requiring conversion, had a significantly higher operative time, blood loss, hospital stay and positive surgical margins, but not a higher overall morbidity rate (35.5% vs. 28.0%) compared with patients operated by VATS (Table 3).
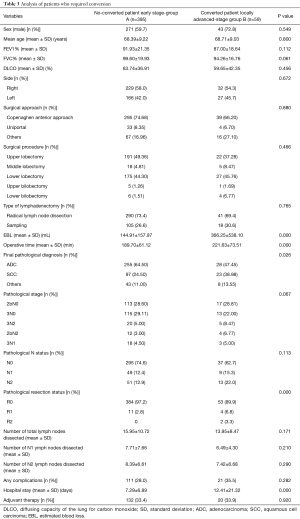
Full table
The mortality rate (1.6% vs. 1.5%), the proportion of patients who suffered from any complication (24.8% vs. 29.1%) and the hospital stay (7.35 vs. 7.96 days) were not statistically different between the two groups (P=0.880, 0.057 and 0.660, respectively). The complication rate was significantly higher in group B (30.4% vs. 37.0%); particularly we observed a higher incidence of hemothorax (1.0% vs. 3.3%) (Table 4).
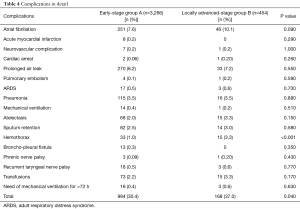
Full table
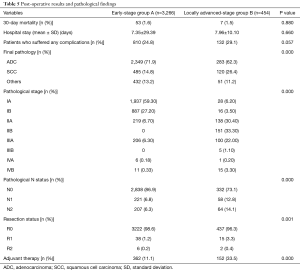
In group B, we observed a larger amount of squamous cell carcinoma (SCC) (26.4%) compared to group A (14.8%). Due to selection bias, the pathological stages were different between the two groups with a higher incidence of advanced stage in group B: 26.8% of patients in group B had metastatic hilar or mediastinal lymph nodes and also 3.7% of patients had positive margins. Furthermore, some adjuvant therapies were administered to 33.5% of patients in group B (Table 5).
Full table
Discussion
VATS-L is recognized to be associated with many advantages compared with lobectomy by thoracotomy. Recent analysis of post-operative outcomes performed on both single institutional series and official databases proposed VATS-L to be superior in terms of length of stay, post-operative pain, preserving pulmonary function, post-operative complications and compliance with adjuvant chemotherapy when compared to open lobectomy (1-6). Moreover, VATS-L has been recommended by National Comprehensive Cancer Network (NCCN) guidelines as the preferred approach for early-stage NSCLC (15).
VATS major pulmonary resections are still considered complex and demanding procedures, characterized by the need of a fine dissection of delicate and vulnerable vascular structures at risk for potential severe and life-threatening bleedings; because of this VATS-L has been used mainly for early-stage NSCLC. With the continuous development of surgical skills and new technical facilities (such as high definition 2-dimension or 3-dimension cameras and displays, endoscopic flexible stapler and retraction instruments), intra-operative technical difficulties have been gradually overcome. However, the role of VATS-L for the treatment of the locally advanced stages of NSCLC is not clear and is not well established; in experienced VATS centres this minimally invasive approach is gaining acceptance even in multimodality treatment of NSCLC (11-13,16).
This study compared outcomes between patients who underwent VATS-L for early-stage and locally advanced-stage NSCLC, using data from the national Italian database (www.vatsgroup.org), and demonstrated that in Italy, VATS-L is a safe approach even for locally advanced-stage NSCLC. In this large retrospective analysis, the two groups did not significantly differ in early outcomes, 30-day mortality (beyond the 2% in both groups) and proportion of patients who suffered from any complication (24.8% vs. 29.1%). The overall complication rate was statistically different between the two groups (30.4% vs. 37.0%) and this datum can be considered quite normal, based on the selection criteria of the two groups; however, the more common complications after thoracic surgery, such as atrial fibrillation, pneumonia, respiratory failure, bleeding requiring transfusion, and prolonged air leak were not statistically different between the two groups. Moreover, even if the overall hospital stay in group B was higher than group A (7.35 vs. 7.96 days), this datum was not statistically significant.
The incidence of surgical complications after resection for locally-advanced NSCLC and/or after neoadjuvant therapy has been reported in the literature to be variable. Hennon and co-authors (11), comparing the outcomes of locally-advanced NSCLC treated by VATS or thoracotomy, observed only one peri-operative death in the VATS group and had a complication rate of 38.9% and 36.8%, respectively. Other similar works about VATS-L versus open lobectomy (12,16-18) showed different complication rates ranging from 25% to 40%, but statistically not higher compared to the open approach. Gonzalez-Rivas et al., comparing early stage (cT1 and cT2) and advanced stage NSCLC treated by uniportal VATS-L, obtained a complication rate of 17.2% and 14.0%, respectively (13). These results demonstrated that VATS-L, in appropriately selected patient operated in experienced centres, is a safe approach even after induction therapy and for locally advanced stage. In addition, VATS-L may hypothetically improve survival because it allows more patients (and more rapidly) to receive adjuvant therapy compared to patients who underwent lobectomy via thoracotomy (5,18).
In our series, 152 patients of group B (33.4%) received some kind of adjuvant treatments. This value can be interpreted with the fact that more complex procedures are not included in our database, often associated with an advanced pathological stage, such as pneumonectomy or bronchial/vascular sleeve resections, planned and performed preferentially through thoracotomy. Hennon et al. (11) showed in his series a similar datum that was significantly higher when compared with the group of resection via thoracotomy (37.2% vs. 5.3%). On the other hand, Chen et al. demonstrated a similar proportion between the two approaches (70% vs. 65%) (16).
The conversion rate was higher (13%), but this datum is easily understandable since the complexity of the performed procedures: large masses could determine a worse handling of the whole lung and often are associated with infiltration of anatomical structures or hilar adenopathy requiring the open approach. Furthermore, the anatomical alterations caused by induction therapies (as calcified lymph nodes or scarring fibrous tissue strongly tightened to pulmonary artery or bronchus) lead to more complex procedures such as a broncho-vascular sleeve resection or a pneumonectomy (9).
Our study demonstrated that the decision of conversion was caused mainly by (I) bleeding not manageable by VATS; (II) an unexpected tumour extension and (III) the presence of hilar calcific lymph nodes precluding a safe dissection. Despite longer procedures and a higher estimated blood loss, the post-operative mortality and complication rates of converted patients were not superior compared with patients operated by VATS, thus demonstrating the limited influence of conversion to open thoracotomy on the post-operative outcomes.
Even if the minimally invasive VATS approach is widely recommended for early-stage NSCLC (15), thoracotomy is still the preferred approach for large tumours and after induction therapies. Our study shows that a substantial portion of patients (14%) with locally-advanced NSCLC can benefit from VATS-L. So, since the lack of specific guidelines, what are the best candidates for minimally invasive resection in case of locally-advanced NSCLC? Our cohort is wide and heterogeneous, but clinical peripheral T2b and T3 tumours without lymph node involvement (stage IIA and IIB) show post-operative outcomes similar to early stages and seem to be the best candidates for VATS-L. Almost 25% of patients had chemotherapy before surgery, but in this cohort, we included different cases that could be differently evaluated on the basis of the clinical experience of the recruiting centre.
The initial doubts about VATS-L oncological adequacy for early-stage NSCLC have been overcome, as demonstrated by several authoritative papers (1-10); the minimally invasive technique and the traditional open technique have proven to be equivalent in terms of overall survival and disease free-survival also for locally advanced NSCLC (10-13). Unfortunately, our study lacks of mid- and long-term survival results; however, we have some valid oncological data such as the extent of lymphadenectomy and the resection margin status. It is well known that an incomplete mediastinal lymph node dissection in NSCLC may result in an incorrect staging and patients would be denied adjuvant treatments and subsequently overall survival may be affected. Our data showed an increased number of lymph nodes dissected for patients with locally-advanced NSCLC, indicating a tendency to a more invasive, aggressive and accurate mediastinal staging in this group. Other authors showed similar results with conventional three-port approach (16) and uniportal approach in single institution series (13).
Finally, this study has several limitations. The database is large and multi-institutional, but the cohort of patients is heterogeneous and non-randomized; it is limited to Italy and the practice patterns may not be representative of other centres outside Italy.
Moreover, our analysis does not include long-term disease-free or overall survival, which are needed to evaluate VATS-L oncological adequacy also for locally advanced NSCLC. Another limitation of our study is the absence of a comparative analysis with an open approach group, since our data comes from a VATS national database.
Concluding, VATS-L for locally advanced-stage NSCLC in Italy seems to be a safe and effective procedure when performed in appropriately selected patients, ensuring peri-operative results similar to those obtained in early-stage tumours. Although conversion rate is higher than in early stage, its influence on post-operative outcomes is limited. Further analyses are needed to compare mid- and long-term survival and confirm the oncological adequacy of minimally invasive approach for locally advanced NSCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870-5. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Gonfiotti A, Bongiolatti S, Borgianni S, et al. Development of a video-assisted thoracoscopic lobectomy program in a single institution: results before and after completion of the learning curve. J Cardiothorac Surg 2016;11:130. [Crossref] [PubMed]
- Watanabe A, Koyanagi T, Ohsawa H, et al. Systematic node dissection by VATS is not inferior to that through an open thoracotomy: a comparative clinicopathologic retrospective study. Surgery 2005;138:510-7. [Crossref] [PubMed]
- Gonfiotti A, Bongiolatti S, Viggiano D, et al. Does videomediastinoscopy with frozen sections improve mediastinal staging during video-assisted thoracic surgery pulmonary resections? J Thorac Dis 2016;8:3496-504. [Crossref] [PubMed]
- Villamizar NR, Darrabie M, Hanna J, et al. Impact of T status and N status on perioperative outcomes after thoracoscopic lobectomy for lung cancer. J Thorac Cardiovasc Surg 2013;145:514-20; discussion 520-1. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Yang CF, Meyerhoff RR, Mayne NR, et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1615-23. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind C. editors. TNM Classification of Malignant Tumours, 7th Edition. Wiley-Blackwell, 2011.
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 8.2017). Available online: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Chen K, Wang X, Yang F, et al. Propensity-matched comparison of video-assisted thoracoscopic with thoracotomy lobectomy for locally advanced non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:967-76.e2. [Crossref] [PubMed]
- Nakanishi R, Fujino Y, Yamashita T, et al. Thoracoscopic anatomic pulmonary resection for locally advanced non-small cell lung cancer. Ann Thorac Surg 2014;97:980-5. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
Cite this article as: Gonfiotti A, Bongiolatti S, Bertolaccini L, Viggiano D, Solli P, Droghetti A, Bertani A, Crisci R, Voltolini L; Italian VATS Group. Thoracoscopic lobectomy for locally advanced-stage non-small cell lung cancer is a feasible and safe approach: analysis from multi-institutional national database. J Vis Surg 2017;3:160.


