Robotic-assisted thoracoscopic surgery thymectomy
Introduction
The most frequent indications for thymectomy are myasthenia gravis (MG) resistant to medical treatment and clinical early stage thymoma. In the past mediastinal surgery was associated with necessity of a maximum exposure, which was accomplished through various invasive open approaches. In the early 1990s many surgical fields, including thoracic surgery, observed the development of minimally invasive techniques, such as the video-assisted thoracoscopic surgery (VATS), which conferred clear advantages: fewer traumas, short hospital stay, increased cosmetic results and preservation of lung function (1). Currently robotic-assisted thoracoscopic surgery (RATS) thymectomy is a valuable alternative to the traditional thymectomy through median sternotomy or VATS approach.
Robotic surgery is better than trans-sternal approach and conventional thoracoscopy?
Many authors endorse the advantages of robotic surgery when compared with trans-sternal approach such as shorter post-operative hospital stay, shorter tube days, lower intra operative blood loss, lower peri-operative complications, better cosmetic results and more delicate dissection (2,3). However to date no randomized trials exist to compare the trans-sternal and robotic approaches for evaluating long-term results (including oncological outcomes). Even if few data demonstrate objective clinical superiority of robotic technology compared with VATS approach (4), in literature are reported advantages of robotic-assisted surgery compared with conventional thoracoscopy such as more precise dissection around vascular and nervous structures thanks to multi articulated instruments and a better vision of the operative field with less risk of incomplete resection thanks to 3-dimensional (3D) high vision (5). Even in this case to date no randomized trials exist to compare the video-assisted and robotic-assisted approaches for evaluating post-operative mortality and long-term results.
Which side approach?
The side to approach the anterior mediastinum is important for a correct surgical excision. We prefer a left side approach for myasthenic patients because it is easier to remove the thymic tissue located in the aortopulmonary window and behind the phrenic nerve (frequent sites of ectopic thymic tissue). Other advantages of the left side approach consist of reduced possibility of phrenic nerve and superior vena cava injury, resection of large left thymic tumours and surgical excision of tumours close to great vessels of the left pericardium (6). We prefer right approach for right thymic tumours because in this case the superior vena cava is an early landmark for dissection and for obese patients because in this situation we have more room in the right thoracic cavity (most of the heart and aorta are not present in the operative field), and so a better visualization with a greater manoeuvrability of instrumentation (7).
Our experience
We report our experience with “da Vinci” robotic system for anterior mediastinal dissection in patients with or without MG and provide data on the surgical results. Between December 2012 and February 2017, a total of 45 patients underwent robotic thymectomy at the Unit of Thoracic Surgery of the Monaldi Hospital in Naples, including 23 male patients (51.1%) and 22 female patients (48.9%), with a mean age of 53.6 and 44.5 years respectively. We performed an anterior mediastinal dissection and used a left side approach in 66.7% of cases. The MG was diagnosed in 40% of cases on the basis of clinical features, repetitive nerve stimulation test and acetylcholinesterase antibody (AbAchR) level. These indications, decided in a multidisciplinary team including thoracic surgeon, neurologist and oncologist, were followed: patients with suspected or proven capsulated thymoma inferior of 5 cm in their large diameter (with no signs of invasion to the surrounding structures) and patients with generalized myasthenic symptoms resistant to medical treatment. The exclusion criteria included: patients with radiological evidence of mediastinal invasion of surrounding structures, inability to perform single lung ventilation, prior mediastinal surgery and pleural adhesions. Surgical technique: the procedure was performed in general anaesthesia and selective intubation with double-lumen orotracheal tube. The patient was placed in a supine decubitus position with left or right emithorax elevated with a long gel roll placed from the hip to the level of the tip of the scapula. We used three port access: first port placed in the anterior axillary line in the 5° intercostal space; second port placed in the 5° intercostal space at mid-clavicular line; last port placed higher in the anterior axillary line, posterior to the edge of the pectoralis major muscle (Figure 1). To obtain a better view of the mediastinum, pleural space was insufflated with carbon dioxide (CO2) at a flow of 6–10 L/min at pressures of 6–8 mmHg. After careful inspection of the entire emithorax, the tissue dissection was carried out. In case of MG the extended thymectomy was performed through en-bloc resection of thymic tissue with surrounding mediastinal fat from the cervical region to the diaphragm extending laterally to both phrenic nerves and also incorporating the mediastinal fat in the aortopulmonary window (additional ectopic thymic tissue may be discovered in this site). Hem-o-lok clips were used for thymic veins closure before electrocauterization. The specimen was removed by endobag. In case of hybrid procedure (RATS followed by thoracotomy approach) for giant thymoma we extended one of the accesses to extract the specimen. The incisions were closed and a single 28 Fr chest tube inserted through one of the port accesses. Histological analysis of the 45 cases revealed: thymoma Masaoka stage I (46.7%), thymic hyperplasia (28.9%), normal thymic tissue (11.2%), thymic remnant tissue (6.6%), thymic cyst (2.2%), bronchogenic cyst (2.2%), solitary fibrous tumour (2.2%). At last follow-up 42 patients were alive and 1 with evidence of mediastinal relapse; three patients were lost. As for patients with MG: 13 (72.2%) showed neurological improvement with reduction of their medications; 2 (11.1%) showed complete stable remission.
Conclusions
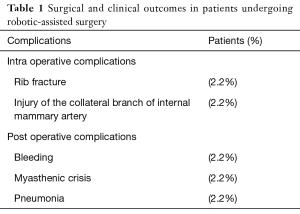
Our experience suggests that the anterior mediastinal dissection with “da Vinci” robotic system surgery is a feasible and safe procedure. In fact in our data base mortality rate was 0% and a low rate of intraoperative and postoperative complications was observed (Table 1). We never had to convert to open surgery. In our opinion this minimally invasive procedure has the important advantage to allow differential diagnosis in a narrow anatomical region as anterior superior mediastinum (for example in case of bronchogenic cyst and solitary fibrous tumour) and we agree with authors that the side of robotic surgery access has to change according to specific indications (6,7). Although there are not objective clinical benefits to use robotic surgery (4), we think that 3D high-vision and articulating instruments are very useful especially in a narrow space such as the anterior mediastinum, allowing the surgeon to perform precise manoeuvres around critical structures for more precise dissection. The better vision of operative field and the use of multi-articulated instruments are the advantages of robotic assisted surgery compared to conventional thoracoscopy, as reported in literature (5). We observed that, with an increased experience, well circumscribed and giant thymomas can be subjected to complete surgical excision using a hybrid procedure that combines RATS with a following thoracotomy (8,9) (Figure 2). Actually the limits of this procedure are its high costs related to maintenance of technology and its long operating room time (10). However, we think that with increased experience the operative time can be reduced, decreasing so operating room costs. Furthermore, today, we need long-term clinical outcomes for anterior mediastinal dissection with robotic and VATS surgery in thymomas and myasthenic patients.
Full table

Acknowledgements
None.
Footnote
Conflicts of Interests: The authors have no conflicts of interest to declare.
References
- Melfi FM, Fanucchi O, Mussi A. Minimally invasive mediastinal surgery. Ann Cardiothorac Surg 2016;5:10-7. [PubMed]
- Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68-73; discussion: e73.
- Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [Crossref] [PubMed]
- Wei B, D'Amico TA. Thoracoscopic versus robotic approaches: advantages and disadvantages. Thoracic Surgery Clinics 2014;24:177-88. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y. Robot-assisted thoracoscopic surgery: current status and prospects. Gen Thorac Cardiovasc Surg 2013;61:127-32. [Crossref] [PubMed]
- Li Y, Wang J. Left-sided approach video-assisted thymectomy for the treatment of thymic diseases. World J Surg Oncol 2014;12:398. [Crossref] [PubMed]
- Deen S, Farivar AS, Louie BE. Thoracic techniques: robotic thymectomy for thymoma. Indian J Surg Oncol 2013;4:132-7. [Crossref] [PubMed]
- Schneiter D, Tomaszek S, Kestenholz P, et al. Minimally invasive resection of thymomas with the da Vinci® Surgical System. Eur J Cardiothorac Surg 2013;43:288-92. [Crossref] [PubMed]
- Curcio C, Scaramuzzi R, Amore D. Hybrid robotic-assisted surgery. Asvide 2017;4:463. Available online:http://www.asvide.com/articles/1780
- Turchetti G, Palla I, Pierotti F, et al. Economic evaluation of da Vinci-assisted robotic surgery: a systematic review. Surg Endosc 2012;26:598-606. [Crossref] [PubMed]
Cite this article as: Curcio C, Scaramuzzi R, Amore D. Robotic-assisted thoracoscopic surgery thymectomy. J Vis Surg 2017;3:162.


