The technique of VATS right pneumonectomy
Introduction
Pneumonectomy as a lung cancer surgery dates back to the first half of the twentieth century (1). During almost 30 years, this was the standard surgical treatment for lung cancer. In the late 1960s, lobectomy was popularized and naturally became the oncological option for resectable primary lung cancer. Additionally, in the last decades the clinical and oncological indications of a pneumonectomy became significantly narrowed and some even consider this surgery as a “disease” itself.
Over the past 30 years, the integration of technology into the medical equipment has led to a new era in thoracic surgery. The constant advances and refinement of operative techniques and surgical equipment have dramatically changed the surgical management of lung cancer. Minimally invasive surgery is now the standard approach to early stage lung cancer resections. In fact, several series confirm the feasibility of complex cases by VATS (2-4), including pneumonectomies.
The goal of this paper is to describe the technical features of a right uniportal VATS pneumonectomy for non-small cell lung cancer its clinical indications, preoperative assessment and postoperative management. This article will not address the completion pneumonectomy.
Indications and contraindications: the current role of pneumonectomy in lung cancer treatment
Pneumonectomy for lung cancer is indicated when a lung sparing procedure does not offer a complete resection. In the case of a central tumor infiltrating the hilar elements, bronchial sleeve, carinal sleeve with or without vascular sleeve lobectomies should be the first surgical option. However, parenchymal, bronchial and vascular margins must all be clear of cancer. Though the above-mentioned resections are technically complex and demanding, several series have confirmed the overall superiority of bronchoplastic procedures when compared to pneumonectomy (5,6). Even in patients with normal lung function, independently of the technique (open or VATS); pneumonectomy should be reserved as the last resource. The reason is simple: pneumonectomy is proven to be related to higher postoperative morbidity and mortality rates if compared to any other lung resection, as well as, it carries a higher potential for a negative impact on postoperative patient’s quality of life (7,8).
The clear indications of pneumonectomy are: central tumors affecting the main bronchus and/or central vascular structures; tumor grossly crossing the fissure (compromising simultaneously the majority of upper and lower lobes); two independent lesions in upper and lower lobes; and in unusual situations of positive interlobar lymph node(s) which infiltrate the main bronchus and lesser resections are not amenable.
The functional pre-operative evaluation consists in pulmonary function test, cardiopulmonary stress test and/or cardiac evaluation, depending on patient comorbidities and medical history. All lung cancer cases are clinically staged according to international lung cancer guidelines (9,10) with chest computed tomography (CT) scan, PET scan and brain MRI or brain CT scan. Once distant metastasis have been ruled out, the lymph node status is the most critical prognostic factor; therefore, every patient candidate to a pneumonectomy must be submitted to invasive mediastinal staging either by EBUS or mediastinoscopy. Occasionally, VATS approach itself can be used as a mediastinal staging tool. Only when the mediastinum is deemed clear of lymph node metastasis, patient can be considered eligible to resection.
On the contrary, if the patient is found to be N2 or clinically unfit for surgery, he will be referred to the oncologist for definitive treatment. In general, we do not recommend neoadjuvant therapy for an upfront pneumonectomy patient. In both scenarios, surgery is excluded from the treatment strategy.
Right sided pneumonectomy: special concerns
The right-side pneumonectomy is known to have more complications and higher mortality rate than the left side one. The cause is unknown, but patients tend to have more bronchopleural fistula and ARDS (5). On the right side, the paratracheal mediastinal dissection is extensive and can contribute to hamper the local vascular supply leading to ischemia to the bronchial stump, therefore increasing the risk of bronchopleural fistula. Thus, a vascularized patch (pericardial fat, parietal pleura or intercostal muscle flap) to reinforce the bronchial stump is strongly suggested. On the left side, however, the bronchial stump retracts within the mediastinum underneath the aortic arch which will work as a natural protection and; therefore, being less prone to fistula.
Minimally invasive pneumonectomy: brief remarks
Minimally invasive pneumonectomy can be performed either by VATS (11,12) or with the robotic platform (13) and has already been described as being feasible even for more advanced stages in lung cancer patients (14,15). In this article, through a case presentation, we will describe and discuss our uniportal VATS technique, as well as the patient’s clinical management.
Compared to other anatomic lung resections, minimally invasive pneumonectomy is still a relatively uncommon procedure either because of its technical challenge or because the overall indications have considerably decreased. The lack of a standardized technique for a VATS pneumonectomy is evident, as some prefer to start with the vascular dissection and others with the lymphadenectomy. Regarding the vascular dissection, again there is variability, some prefer to transect the artery first, others the veins. Independently, published series indicates that VATS pneumonectomy is a safe procedure, associated with less pain (3) and at least equivalent oncologic early outcomes, morbidity and mortality rates compared to thoracotomy (3,16). In fact, overall survival is also comparable (4). More studies with longer follow-up are awaited to clarify the risks and the benefits offered by a VATS pneumonectomy.
Case description and pre-operative workup
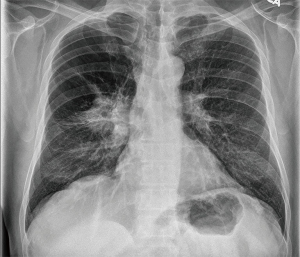
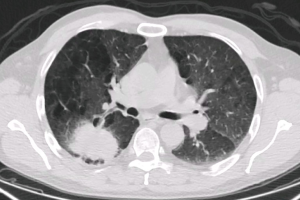
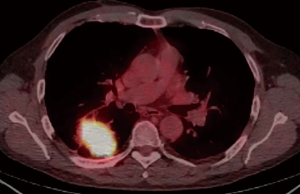
A 59-year-old male active smoker presented a right central lung mass identified in a chest X-ray, during an acute respiratory infection episode. Despite antibiotic treatment, there was no improvement in the X-ray (Figure 1). Further investigation with chest CT scan revealed advanced pulmonary emphysema and an irregular peripheral mass in the right upper lobe (RUL) of 54 mm × 50 mm, crossing the fissure towards the right lower lobe (RLL) (Figure 2). There was no evidence of hilar or mediastinal lymph nodes enlargement. Otherwise, the CT scan was normal. The positron emission computed tomography (PET-CT) scan (Figure 3) showed a high 18-FDG (fluorodeoxyglucose) uptake by the tumor (SUV =13.5) and a minor uptake on an ipsilateral hilar lymph node (SUV =2.2). No other lesions have been identified at the PET-CT scan. Brain MRI was normal. Flexible bronchoscopy was performed and a transbronchial biopsy confirmed the presence of a squamous cell carcinoma of the RUL. A staging EBUS with biopsy of stations 2R, 4R, 10R, 7, 2L and 4L confirmed the absence of mediastinal metastasis. The patient’s final clinical staging was cT2bN0M0—stage IIA, according to the 7th TNM (17). Considering the new 8th edition of the same TNM classification (18), the clinical staging is cT3N0M0—stage IIB.
Patient’s spirometry revealed a FEV1 =2.98 L (91%) and a DLCO =80%. Cardiopulmonary exercise test demonstrated a VO2max =27 mL/kg/min, with a maximum heart rate of 150 (89% of predicted). Accordingly, the patient was considered fit for surgery.
The surgery: anesthetic considerations and operative technique
The patient is under general anesthesia with a double lumen tube for single lung ventilation. Two peripheral venous lines and an arterial line were installed. For minimally invasive surgeries, we do not use epidural nor urinary catheters. During surgery, intravenous fluids administration is restricted to a minimum.
The patient is placed in full left lateral decubitus with the hip flexed. Preemptive analgesia with intercostal nerve block and blockage at the incision is performed. The incision has about 4 cm and is placed at the 5th intercostal space. A soft tissue retractor is placed in order to keep the incision open and facilitate the instrumentation. We routinely use a 5 mm camera with a 30-degree angle and bi-articulated VATS dedicated instruments. Endoscopic staplers are used for vascular, bronchial and fissure transection. When appropriate, polymer clips, harmonic scalpel or other energy device can also be applied to ligate vessels smaller than 5–7 mm. For the lymph node dissection, several surgeons also prefer to use sealing devices.
Main surgical steps of the procedure (Figures 4-15)












We start the oncologic lung resections by performing complete visual inspection of the pleural cavity to confirm resectability. In this particular case, due to severe pleuropulmonary adhesions, it was impossible to perform a full examination upfront. Figure 4 illustrates the take down of the adhesions. We combine the use of cautery, energy device and blunt dissection to achieve full release of the lung.
Once the adhesions have been freed enough to allow safe retraction, the lung is pulled anteriorly and cranially to expose the costophrenic angle. The dissection is started at the level of the inferior pulmonary ligament on the pleural reflection and progresses upwards towards the azygos vein, as shown in Figure 5. As this dissection proceeds, the lymph nodes of station #8 and #9 are removed (para-esophageal and pulmonary ligament lymph node stations) and sent for frozen analysis. Along with the lymphadenectomy is also productive to dissect the vessels like the inferior pulmonary vein at this level. We do not however, recommend the ligation of any vascular structure before confirming that the resection will take place.
At the subcarinal level, a more extensive lymphadenectomy is performed and can be seen on Figure 6. In our personal experience, the majority of pneumonectomy patients will have undergone a mediastinoscopy previous to the resection, in which case, we will move straight to the vascular dissection. In this particular case however, the mediastinal staging was performed exclusively with the EBUS, so through the VATS approach the lymphadenectomy was done first.
After the subcarinal dissection shown in Figure 6, Figure 7 shows an advance of the dissection towards the superior portion of the hilum to fully release the pleural reflection. The dissection continues, advancing towards the anterior vessels of the hilum. For this part, the structures are better exposed by retracting the lung posteriorly and caudally. The table is turned laterally away from the surgeon so the lung can lay down easier as necessary, enhancing the exposure of the vascular structures.
Our preferred approach to pneumonectomy is the control the main pulmonary artery (PA) first. On the left side, the PA is long and extra-pericardial, allowing an easier control. On the right side, however, the shorter PA and emerging tronchus anterior branch can limit full exposure of the base of the right PA trunk. To facilitate a complete and safe access to the PA, we developed a two-step approach. First, we staple the tronchus anterior (as seen in Figure 8) and second, we staple the RUL vein (as shown in Figure 11). Once both of these vessels are transected, the access to the remaining PA is easily achieved.
Next to the tronchus anterior dissection, we perform the right paratracheal lymph nodes dissection. The access to the stations #4R and #2R is performed by creating a tunnel underneath the azygos vein and the mediastinal pleura or by opening the mediastinal pleura directly, cranially to the azygos vein and posterior to the superior vena cava. The superior vena cava must be retracted away from this dissection. This lymph node dissection is well demonstrated in Figure 9.
In all pneumonectomies, performing a good paratracheal and subcarinal lymph nodes dissection are important steps to expose the tracheobronchial angle and the carina, facilitating then the full exposure and isolation of the right main bronchus, in order to provide the shortest bronchial stump possible. This bronchial isolation is illustrated in Figure 10. On the other hand, it is important to balance the dissection on the proximal main bronchus to prevent bronchial stump ischemia and reduce the risk of bronchopleural fistula.
As previously mentioned, the control of the PA first is our primary objective in this type of procedure. In a stepway fashion, when transecting the truncus anterior and the superior pulmonary vein we obtain full exposure of the base of the remaining right PA. In fact, these are simple and straightforward steps that do not require any major dissection or delicate maneuver. Once the tronchus anterior is stapled, there is a better and safer angle to dissect and transect the venous branches for middle and upper lobes. With the help of a vessel loop the vein is completely released from its adhesions, becoming fully accessible for stapling. Figure 11 shows the dissection, isolation and stapling of the superior pulmonary vein.
The benefits of doing the PA first in the uniportal technique are:
- The most difficult and dangerous step of the pneumonectomy is performed first;
- Once the artery is transected there is no pulmonary congestion and swelling, what will decrease the risk of bleeding and facilitate the extraction of the specimen through the single-port incision;
- The subsequent steps of the operation; both veins and the bronchus become easier to dissect. It can also be easily seen on the same Figure 11.
Figure 12 contemplates the isolation and division of the interlobar portion of the PA. Here, just as with the superior vein, it is clearly demonstrated how safer and easier becomes the handling of the remaining PA when the truncus anterior branch is already stapled/divided.
Once the PA is divided, we proceed to the ligation of the inferior pulmonary vein, leaving the right main bronchus for last. Both of these steps are well demonstrated on Figure 13. If necessary, further bronchial dissection can be carried out to provide the shortest bronchial stump possible. It is interesting to see in this figure how the retraction of the main bronchus upwards can help the exposure of its most proximal portion. Perioperative flexible bronchoscopy can be used to ensure the correct bronchial stapling.
Once the pneumonectomy is completed, the specimen is removed using a protective bag. The removal of such a big specimen through a small incision can be a tricky task. Correct alignment of the lung during its extraction and the use of continuous rotational movements and traction in each edge of the bag are some tips. In some cases, is necessary to enlarge the skin and/or the intercostal muscle incisions in a few millimeters. Frozen section analysis is performed for bronchial and vascular margins, as well as in any other margin suspected to be compromised by the tumor.
After lung removal from the pleural cavity, the hemostasis is verified and the bronchial stump is tested to rule out air leaks. At last, the bronchial stump is reinforced with a pleural or muscle flap to reduce the risk of bronchopleural fistula, as seen in Figure 14.
At the end of the procedure, we perform an intercostal nerve block with local anesthetic solution from the third to the ninth intercostal spaces, in order to provide immediate postoperative analgesia. A 24 French sized chest tube is inserted through the posterior end of the surgical incision as shown in the Figure 15. All patients receive PCA pump (patient-controlled analgesia device), for intravenous doses of hydromorphone.
Post-operative management
The concept of “minimally invasive surgery” must be expanded to “minimally invasive treatment”, a more appropriate terminology that embraces not only the surgery itself, but also a number of pre and postoperative measures that are closely aligned to the minimally invasive philosophy.
Following this rationale, we believe that patients that undergo VATS procedures should be managed by early physiotherapy, early ambulation and early oral feeding, as well as intravenous liquid restriction and adequate pain control.
In pneumonectomy cases, we choose to keep the patients in the intensive care unit (ICU) for the first 48 hours. Patients submitted to lesser resections are usually admitted in the step-down unit. The chest tube is removed on the first postoperative day once bleeding and chylothorax are ruled out. This patient was discharged home on the fifth postoperative day with no surgical complications.
The final pathologic report confirmed a squamous cell carcinoma with a subcarinal metastasis (station #7). The final pathologic staging was then pT2bN2M0—stage IIIA, according to the TNM Lung cancer staging system. Due to the mediastinal lymph node metastasis, the patient was referred to medical oncology for adjuvant chemotherapy and radiotherapy.
The patient has regular follow-up at the clinic. Adjuvant treatment was completed uneventfully. During his last visit, at one year after surgery, the patient was in good health conditions, with no respiratory complaints, sporadic low-grade pain at the site of the surgery—easily controlled by regular analgesics—and referring good quality of life.
Conclusions
Uniportal right-side VATS pneumonectomy is a complex procedure yet feasible and safe when carried out by an experienced minimally invasive thoracic surgeon. The literature is limited, but suggests that minimally invasive pneumonectomy is at least equivalent to open thoracotomy regarding the surgical and oncologic outcomes and postoperative quality of life. Nevertheless, more studies are still necessary to define the role of VATS pneumonectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Graham EA, Singer JJ. Successful removal of the entire lung for carcinoma of the bronchus. JAMA 1933;101:1371-4. [Crossref] [PubMed]
- Sahai RK, Nwogu CE, Yendamuri S, et al. Is thoracoscopic pneumonectomy safe? Ann Thorac Surg 2009;88:1086-92. [Crossref] [PubMed]
- Liu Y, Gao Y, Zhang H, et al. Video-assisted versus conventional thoracotomy pneumonectomy: a comparison of perioperative outcomes and short-term measures of convalescence. J Thorac Dis 2016;8:3537-42. [Crossref] [PubMed]
- Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg 2010;89:S2102-6. [Crossref] [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites of recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Rendina EA, Venuta F, de Giacomo T, et al. Parenchymal sparing operations for bronchogenic carcinoma. Surg Clin North Am 2002;82:589-609. vii. [Crossref] [PubMed]
- Vannucci F. Tratamento cirúrgico do carcinoma pulmonar. In: Figueiredo EM, Correia MM, de Oliveira AF. editors. Tratado de Oncologia. Rio de Janeiro: Revinter, 2013:655-79.
- Leo F, Scanagatta P, Vannucci F, et al. Impaired quality of life after pneumonectomy: Who is at risk? J Thorac Cardiovasc Surg 2010;139:49-52. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines on Non-Small Cell Lung Cancer. Version 9. 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Chen HW, Du M. Video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2015;7:764-6. [PubMed]
- Gonzalez-Rivas D, Delgado M, Fiera E, et al. Uniportal video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2013;5 Suppl 3:S246-52. [PubMed]
- Spaggiari L, Galetta D. Pneumonectomy for lung cancer: A further step in minimally invasive surgery. Ann Thorac Surg 2011;91:e45-7. [Crossref] [PubMed]
- Demmy TL. Video-assisted thoracoscopic extrapleural pneumonectomy for malignant mesothelioma. Ann Cardiothorac Surg 2012;1:533. [PubMed]
- Gonzalez-Rivas D, Fiera E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Nagai S, Imanishi N, Matsuoka T, et al. Video-assisted thoracoscopic pneumonectomy: retrospective outcome analysis of 47 consecutive patients. Ann Thorac Surg 2014;97:1908-13. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Vannucci F, Vieira A, Ugalde PA. Release of pleural adhesions. Asvide 2018;5:012. Available online: http://asvidett.amegroups.com/article/view/22334
- Vannucci F, Vieira A, Ugalde PA. Opening the posterior pleural reflection and initial dissection of the inferior pulmonary vein. Asvide 2018;5:013. Available online: http://asvidett.amegroups.com/article/view/22335
- Vannucci F, Vieira A, Ugalde PA. Subcarinal mediastinal lymph node dissection. Asvide 2018;5:014. Available online: http://asvidett.amegroups.com/article/view/22336
- Vannucci F, Vieira A, Ugalde PA. Further dissection of pleural reflection and release of additional apical adhesions. Asvide 2018;5:015. Available online: http://asvidett.amegroups.com/article/view/22337
- Vannucci F, Vieira A, Ugalde PA. Hilar dissection: pulmonary artery dissection, isolation and stapling (truncus anterior branch). Asvide 2018;5:016. Available online: http://asvidett.amegroups.com/article/view/22339
- Vannucci F, Vieira A, Ugalde PA. Paratracheal mediastinal lymph node dissection. Asvide 2018;5:017. Available online: http://asvidett.amegroups.com/article/view/22340
- Vannucci F, Vieira A, Ugalde PA. Dissection of the right main bronchus. Asvide 2018;5:018. Available online: http://asvidett.amegroups.com/article/view/22341
- Vannucci F, Vieira A, Ugalde PA. Superior pulmonary vein: dissection, isolation and stapling. Asvide 2018;5:019. Available online: http://asvidett.amegroups.com/article/view/22342
- Vannucci F, Vieira A, Ugalde PA. Further arterial dissection, isolation and stapling (remaining interlobar portion of the pulmonary artery). Asvide 2018;5:020. Available online: http://asvidett.amegroups.com/article/view/22344
- Vannucci F, Vieira A, Ugalde PA. Right main bronchus and inferior pulmonary vein: final isolation and stapling. Asvide 2018;5:021. Available online: http://asvidett.amegroups.com/article/view/22345
- Vannucci F, Vieira A, Ugalde PA. Pleural patch over the bronchial stump and complementary lymph node dissection. Asvide 2018;5:022. Available online: http://asvidett.amegroups.com/article/view/22346
- Vannucci F, Vieira A, Ugalde PA. Anesthetic multilevel intercostal nerve block and chest tube positioning. Asvide 2018;5:023. Available online: http://asvidett.amegroups.com/article/view/22347
Cite this article as: Vannucci F, Vieira A, Ugalde PA. The technique of VATS right pneumonectomy. J Vis Surg 2018;4:11.