Small incisions, major complications: video-assisted thoracoscopic surgery management of intraoperative complications
Introduction
Video-assisted thoracoscopic surgery (VATS) represents the gold standard approach for lung cancer not only for the treatment of early stage lung cancer but also for more advanced disease, depending on the surgeon’s surgical skill. VATS is widely considered a safe and effective surgical technique with a low post-operative complication rate. The most common intraoperative complications, that lead to conversion, are featured by massive bleeding, airway injuries, and tough adhesions.
Patient selection and workup
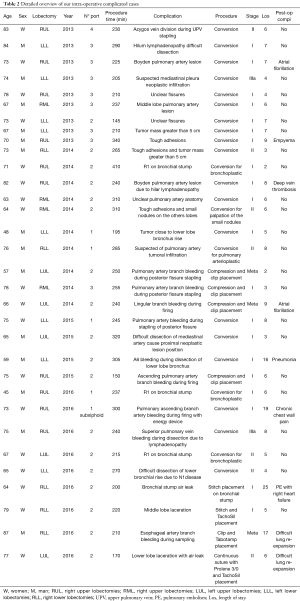
This study is the result of an institutional retrospective review of all the patients that underwent VATS Lobectomy from 2013 to 2016. The aim of this study is to report our department VATS lobectomy intra-operative complications and to analyse their management and the influence that they have on the conversion rate and on the patient’s outcome in terms of post-operative complications and length of stay. We considered several variables such as patient’s age at time of surgery, duration of procedures, different techniques adopted in terms of ports’ number and approach, preoperative patients’ selection and assessment, conversion rate, different intraoperative injuries that lead to conversion, postoperative length stay and complication. Data were collected using the hospital’s database (G2 software) and medical records for missing data. Data were managed using Apple Numbers 4.2 Version and analyzed using SPSS for Mac 24.0. Differences among the categorical data were analyzed with either Fisher's exact test or chi-square comparison. Moreover, we performed a univariate analysis with Cox regression test to identify the responsible variables in terms of conversion rate and the relationship between conversion and patient’s outcome. All P values lower than 0.05 were considered statistically significant. 180 patients underwent VATS lobectomy, mostly for lung cancer, with a median operation time of 224 minutes (120–430 minutes). Twentyfour cases underwent conversion, 12 cases had intra-operative bleeding, 3 cases had airways injuries, 3 cases had other injuries, the remaining cases showed tough adhesions and hilar lymphadenopathy. Among all the VATS performed, 56 were right upper lobectomies (RUL), 18 were middle lobectomies (ML), 38 were right lower lobectomies (RLL), 35 were left upper lobectomies (LUL) and 34 were left lower lobectomies (LLL). The postoperative complications observed were: re-operation (1%), prolonged air leaks (12%), acute coronary syndrome (0.6%), atrial fibrillation (7.8%), atelectasis (1%), empyema (0.6%), pneumonia (4.4%), residual pleural space (5.6%), chest tube repositioning (3.3%), other minor complications (14%). The preoperative diagnosis was performed in 53% of cases by trans-bronchial needle aspiration (TBNA) bronchoscopy or computed tomography (CT) scan needle biopsy. Patients operated for lung cancer (98%) underwent lobe specific sampling in 170 cases (94%) and systematic lymphadenectomy in 10 cases (6%). We observed a significant correlation between cN1-cN2 with SUV >4 at positron emission tomography (PET), and pathological evaluation after lymphadenectomy (pN) (P<0,001). At pathological evaluation, 155 cases were pN0 (86%), 15 cases pN1 (8%) and 10 cases pN2 (6%). We observed adenocarcinoma in 99 cases (55%), squamous cells carcinoma in 40 cases (22%), carcinoid in 15 cases (8%) and in the remaining 23 cases (13%) other primary lung neoplasms. Despite pleural adhesions were not responsible for post-operative prolonged air leaks, they were associated to a higher procedure time and higher conversion rate (P<0.010). Seventy-years older patients were not associated with a higher conversion rate, while these patients showed a significantly increased risk of post-operative complications (P<0.018, RR =1.8). In our experience, LLL were statistically correlated to a higher conversion’s risk (P<0.006), instead RUL were significantly related to a higher postoperative complication’s rate (P<0.002). The main factors leading to conversion were intraoperative bleeding and airway injuries (P<0.001). The conversion’s rate decreased from 33% in 2013 to 5.5% in 2015, and was 10% in 2016. At the same time, the decreased conversion rate corresponded with increased VATS management of the intraoperative complications (0% in 2013; 44% in 2016). We believe that the small increase of conversion rate in 2016 is related to challenging cases enrolled to surgery (1) (Table 1, Figure 1). The intraoperative complications occurred from 2013 to 2016 are summarized in Table 2.

Full table
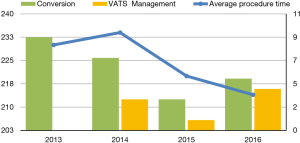
Full table
Pre-operative preparation
Patients underwent a pre-operative assessment with CT-scan, PET in 74% of cases, bronchoscopy and endobronchial ultrasound (EBUS) if evidence of cN1 or cN2 at CT-scan or PET with SUV>4, pulmonary functional tests (FEV1, FVC and Diffusing Capacity of the lungs for carbon monoxide (DLCO) integrated by VO2max in case of Forced expiratory volume (FEV1) and DLCO <70%, according to ESTS lung cancer guidelines (2). 96 patients (53%) had histologic diagnosis at time of surgery, in the other cases, intra-operative diagnosis were performed by wedge resection or core needle biopsy of the pulmonary lesion, and intra-operative histopathological evaluation was required. Patients who underwent VATS segmentectomy, wedge resection and all open surgery were excluded from this study.
Equipment preference card
Patients were positioned in lateral decubitus, the utility incision was 3.5–5 cm wide, no ribs spreading, the inferior port was performed with direct visualization and placed anteriorly the diaphragm’s free margin, usually in the 8th intercostal space on the posterior axillary line. We use 30-degrees camera 10 mm and Scanlan surgical instruments. No trocars or soft tissue devices were employed. The analgesic therapy was administrated in VATS at the end of the procedure through multiple intercostal nerves blocks with Rupivacain 0.75% 10 mL. One chest tube (24 Fr) was placed in the second operative port. In case of conversion, we performed anterolateral thoracotomy and placed two chest tubes (28 Fr).
Procedure
All VATS lobectomies were performed by using the anterior approach according to Copenhagen technique. In the early period of our VATS program, VATS lobectomies were performed using 3 or 4 ports and only patients with stage I, complete fissures and no tough adhesions, were considered eligible to VATS procedures. In fact, in the very beginning of our experience, the presence of adhesion was considered enough to perform conversion. With increased surgeon’s VATS skills, VATS lobectomies were performed with 2 ports and, later were switched to one port. Because of a significant correlation between uniportal technique and post-operative air leak associated with the need to replace the chest drainage (P<0.008), we have abandoned this technique in favor of biportal VATS lobectomy.
Role of team members
Three surgeons were involved in this study: Dr. A Morelli, Udine Thoracic Department’s chief, Dr. G Masullo, Dr. F Londero, and Dr. W Grossi. All surgeons attended an international VATS program and they completed their VATS lobectomy learning curve (25 cases) in less than 12 months. Dr. W Grossi has collected and reviewed the data from the Institute’s database and he has elaborated them with SPSS.
Dr. F. Londero and Dr. G. Masullo collaborate in collecting data and they helped writing this article. Dr. A. Morelli reviewed the article and gave his authorization to publish it.
Post-operative management
Patients who underwent VATS lobectomy came back to ward or intensive care unit after surgery according to the anesthesiologist indications. All patients had one chest tube (24 or 28 Ch) at −20 cm H2O suction for at least 24 hours. On the day of surgery and on first post-operative day, all patients had blood test and chest X-ray. Most of the patients underwent a quick mobilization on armchair on day zero and on the postoperative day one they started walking and doing respiratory physiotherapy. Chest tube was removed in cases of absence of air leak, and patients were discharged the day after according to their degree of mobilization. The analgesic therapy was submitted by morphine, nonsteroidal anti-inflammatory drugs (NSAIDs) and ondansetron by intravenous elastomeric pump and shifted to oral therapy the day before the discharge.
Tips, tricks and pitfalls
A perfect knowledge of anatomical structure and possible anomalies is mandatory to prevent intra-operative complications (3). In the biportal approach, the correct position of the inferior port is crucial. This port should be performed in the eighth intercostal space, across the line just anterior to the tip of the scapula. In fat and short patients, it is mandatory to perform this port under direct visualization to prevent incorrect position in case of diaphragm elevation. We use the inferior port to retract the lung during hilar’ structures’ dissection, and to make sure that the stapler creates a perfect angle for the dissection of the hilarhilar’s structures; to do this, we move the ring clamp from the inferior port to the utility incision retracting the lung up and backward. It goes without saying that if the inferior port is placed in a wrong place, the VATS procedure becomes challenging.
General recommendations:
In case of intra-operative bleeding the first thing is to keep calm, do not panic and press on the bleeding vessel, and wait for haemodinamic stabilization by the anesthetist (4-6); if bleeding cannot be controlled by compression, and the patient is still stable, it could be useful to perform an additional port specifically for sponge compression, so that it becomes possible to carry out the VATS lobectomy (Figure 2). In case of conversion, we routinely perform an anterolateral thoracotomy.

Conclusions
VATS lobectomy is a safe and effective surgical procedure in terms of length of stay, patient’s outcome and oncological radicality. Despite this technique is characterized by small incisions, it could cause major and potentially fatal complications, if not promptly managed. The best way to prevent them is to make a correct patient’s selection and pre-operative assessment, following a specific VATS program at the beginning of VATS surgical experience, and choosing non challenging cases in the early period of the learning curve. In our experience, the uniportal approach was significantly correlated with several cases of chest tube repositioning for air leaks (8). For this reason, we shifted to the biportal approach, as it appeared to be safer in terms of post-operative complications. The most common intra-operative complications that led to conversion were bleeding, airway injuries and adhesion at the beginning of VATS program (9-10). There was no statistical difference in terms of conversion rate between skilled surgeons and learning curve period. It is our opinion that the intra-operative management depends on the surgeon’s VATS skills. Conversion should not be considered a failure of VATS, but rather a surgical choice aiming to patient’s safety. The converted VATS lobectomies are not related to increased post-operative complication rate. In our experience, the LLL was the most challenging VATS procedure and subjected to higher conversion rate. RUL was instead associated with higher post-operative complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Konge L, Petersen RH, Hansen HJ, et al. No extensive experience in open procedures is needed to learn lobectomy by video-assisted thoracic surgery. Interact Cardiovasc Thorac Surg 2012;15:961-5. [Crossref] [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27. [Crossref] [PubMed]
- Ismail M, Swierzy M, Nachira D, et al. Uniportal video-assisted thoracic surgery for major lung resections: pitfalls, tips and tricks. J Thorac Dis 2017;9:885-97. [Crossref] [PubMed]
- Mei J, Pu Q, Liao H, et al. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg Endosc 2013;27:530-7. [Crossref] [PubMed]
- MTP for Hemorrhagic Shock. American Society of Anesthesiologists Committee on Blood Management. Available online: shockhttps://www.asahq.org/resources/resources- from-asa-committees/committee-on-patient-blood-management/mtp-for-hemorrhagic-
- Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: Catastrophic intraoperative complications. J Thorac Cardiovasc Surg 2011;142:1412-7. [Crossref] [PubMed]
- Grossi W, Masullo G, Londero F, et al. Inferior pulmonary artery bleeding during VATS LLL. Asvide 2018;5:028. Available online: http://asvidett.amegroups.com/article/view/22361
- Fernández Prado R, Fieira Costa E, Delgado Roel M, et al. Management of complications by uniportal video-assisted thoracoscopic surgery. J Thorac Dis 2014;6:S669-73. [PubMed]
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5:S182-9. [PubMed]
- Puri V, Patel A, Majumder K, et al. Studying intraoperative conversions from video-assisted thoracoscopic surgery (VATS) lobectomy to open thoracotomy. J Thorac Cardiovasc Surg 2015;149:55-62.e1. [Crossref] [PubMed]
Cite this article as: Grossi W, Masullo G, Londero F, Morelli A. Small incisions, major complications: video-assisted thoracoscopic surgery management of intraoperative complications. J Vis Surg 2018;4:12.


