Robotic vascular resections during Whipple procedure
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related death in the United States. Surgical resection with negative margins is the only curative chance for patients with no evidence of metastasis at diagnosis. Several studies have shown similar outcomes for patients with loco-regional disease as compared to patients with resectable pancreatic cancer, if R0 resections are attained at the time of surgery (1-3). These outcomes are generally seen with the addition of neoadjuvant therapies to the treatment algorithm (4-6).
Pancreatic surgery with major venous resection remains a topic of controversy. However, more institutions are offering this surgery to carefully selected patients. Given the added complexity of pancreatic resection with venous reconstruction, the initial surgical approach was open surgery. However, minimally invasive approaches have recently been applied as selected centers have gained experience in robotic or laparoscopic pancreatic resections (7,8). Various institutional reviews have shown that minimally invasive surgery for pancreatic resections that treat borderline resectable or locally advanced pancreatic tumors is both safe and feasible (9-14). This article focuses on the technique of robotic Whipple procedure with concomitant vascular resection used at our institution.
Preoperative workup
A triphasic, contrast-enhanced computed-tomography scan of the abdomen and pelvis is attained to rule out metastatic disease and evaluate the pancreatic tumor and its relationship to the peri-pancreatic vessels. Next, an endoscopic evaluation is performed with ERCP +/− stenting and brushings, and endoscopic ultrasound (EUS) to delineate the extent of vascular involvement and to attain a biopsy for tissue diagnosis. Chemotherapy is recommended for all patients with preoperative evidence of abutment/encasement of peri-pancreatic vessels. We measure CA 19-9 levels before (once patients have normal total bilirubin) and after neoadjuvant therapy. CA 19-9 reduction after neoadjuvant therapies has been associated with increased rates of R0 resection, histopathological response and survival (15). CA 19-9 response, in the absence of radiographic response to neoadjuvant therapy, may at times help decide if a patient is a candidate for surgical exploration, as long as the venous involvement appears amenable to reconstruction. Specifically, a good inflow and outflow target with a relatively short segment are required. All cases are considered individually and discussed in a multidisciplinary tumor board. Patients are not excluded from being offered minimally invasive resection based on age, body mass index (BMI), or comorbidities; however, prior extensive abdominal surgery, particularly prior pancreatic or liver resection, is considered a relative contraindication. The only absolute contraindication to robotic PD at our institution is vascular encasement of a long segment of the portal vein (PV) or superior mesenteric vein (SMV), which would likely require an interposition graft. We typically use the internal jugular vein as our conduit for interposition grafts. The ergonomics of the robot docked over the patient’s head can make this harvest potentially challenging.
Procedure
Anesthesia preparation
Prior to surgery, patients take a bowel prep and are encouraged to carbohydrate-load. Additionally, they are allowed to have clear liquids until two hours prior to surgery. All patients are considered for our institutional enhanced recovery pathway after surgery (ERAS) protocol using multimodal analgesia, minimizing IV opioids and intra-operative goal-directed fluid management. Like other major abdominal procedures, patients receive a dose of prophylactic subcutaneous heparin prior to induction and wear pneumatic mechanical compression boots. Preoperative antibiotics are administered within one hour of incision and re-dosed as indicated. Hemodynamics are monitored with an arterial line, +/- central venous catheter, and Foley. An oral gastric tube is placed after intubation and removed during surgery.
Port placement
Over time several minor modifications have been made to our standard robotic PD (16-18). A split leg table is utilized to allow access to the abdomen for an assistant surgeon. Entry into the abdominal cavity is achieved via a one cm incision in the left upper quadrant using an optical separator trocar and a 0-degree 5-mm scope. The abdominal cavity is then insufflated with CO2 gas. The abdomen is inspected for evidence of carcinomatosis or metastatic disease. The remaining ports are then placed. A 12-mm port is placed two fingerbreadths above and to the right of the umbilicus, two robotic 8-mm ports are placed in the right upper quadrant, a 5-mm port is placed in the right lower quadrant, a 12-mm port in the left lower quadrant and a 5-mm port in the anterior axillary line on the left side of the abdomen. Typical port placement is depicted in Figure 1. A liver retractor is then placed through that anterior axillary line port with a Mediflex.
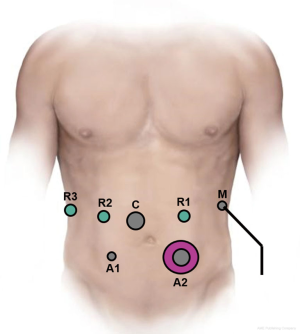
Laparoscopic preparation
The ligament of Treitz is identified and the bowel is then traced about 80 cm distal to it. This segment of the small bowel is then tacked down to the stomach in an antecolic, isoperistaltic fashion using an EndoStitch. This will be the site for the gastrojejunostomy later on during the reconstruction. The 12-mm ports are closed with a figure of “8” stitch. The patient is then placed in steep reverse Trendelenburg with right side up and the robot is then docked.
The resection
The dissection begins by accessing the lesser sac through the greater omentum below the gastroepiploic pedicle. The distal half of the greater curvature omentum is mobilized. Then the avascular plane between the colon and the duodenum is separated, allowing for mobilization of the hepatic flexure followed by a Cattell–Braasch maneuver. The colonic mesentery is dissected off Gerota’s fascia and rolled over as well, until the duodenum is identified. A Kocher maneuver is then performed all the way from the foramen of Winslow to the Ligament of Treitz, taking down all the fibers until the Inferior Vena Cava and the left renal vein are identified. All the fibers of the Ligament of Treitz are dissected until the jejunum can be delivered through to the right upper quadrant.
For cases where venous resection is anticipated, an extensive colonic mobilization is performed such that the root of the mesentery can be mobilized after transection of the SMV. An outline of the superior mesenteric artery (SMA) from the aorta can also be appreciated at the conclusion of this maneuver. The jejunum is transected approximately ten cm distal to the Ligament of Treitz using a stapler. The bowel mesentery is then taken with an energy device until the uncinate process is reached.
Next, the lesser sac is opened up through the pars flaccida, the right gastric and gastroepiploic arteries are ligated and the stomach is divided with a stapler. The oral gastric tube is removed prior to stomach transection. The dissection continues along the superior pancreas and into the porta hepatis. The common hepatic artery is exposed following removal of the common hepatic lymph node. The gastroduodenal artery (GDA) is then identified and test clamped to confirm blood flow in the common hepatic artery. Flow is assessed visually or with color flow Doppler with robotic ultrasound probe. Once flow is verified, the GDA is stapled and marked with a metal clip. Dissection continues until the common bile duct (CBD) and portal vein are exposed. The CBD is then divided with a stapler. Next, the SMV is dissected off the inferior pancreas and a tunnel is created. Finally, the pancreas is transected with the hot scissors halfway through the gland and the duct is cut with the cold scissors technique.
Vascular dissection
For cases where a vein resection is anticipated an “artery-first” approach is used, staying lateral to the porto-mesenteric venous junction, dissecting inferiorly to superiorly. We often accomplish this through a “hanging maneuver”, whereby the SMV above the first jejunal branch, splenic and portal vein are isolated and looped. The third robotic hand can then grasp all three of the vessel loops mobilizing the SMV and PV to the right, allowing for dissection of the SMA. This maneuver helps free the peripancreatic tissues near the SMA allowing for full assessment of the extent of venous resection required. If venous involvement is marginal, we may transect using a microCutter stapler in a tangential fashion or resection with re-approximation by primary venorrhaphy. If abutment is moderate (45–180 degrees of involvement), our preference is to transect and reconstruct using a pericardial bovine patch, as shown on the attached video. Patients with encasement greater than 180 degrees for an extended segment are scheduled for open procedures and reconstructed using Internal Jugular Vein grafts. While there is no technical reason preventing these procedures from being performed with the robotic platform, the logistics and timing of the conduit harvest, concurrent with the steep reverse Trendelenburg positioning are a few challenges that have kept this approach from gaining traction.
Once ready to resect, the patient is heparinized (usually single IV bolus of 3,000 units of heparin) with an unfractionated bolus. The portal vein, splenic vein and SMV are all circumferentially dissected and encircled with vessel loops. Small branches, including the coronary or inferior mesenteric vein, are potentially ligated if within our clamps. Laparoscopic bulldog clamps are placed across the three venous tributaries by the bedside assistant. Next, tangential resection or partial venectomy is performed according to the extent of involvement as described above. Typically we use a 5-0 prolene suture for primary repair or for vascular patches. In the case of a patch, it is sewn in a “diamond” formation and infused with heparinized saline prior to unclamping as demonstrated in video (Figure 2). A cholecystectomy is then performed and the specimens extracted via the left lower quadrant.

Reconstruction
The reconstruction then begins with a two-layer, end-to-side, duct-to-mucosa modified Blumgart pancreaticojejunostomy. First, 2-0 silk transpancreatic horizontal mattress sutures are placed to secure the pancreatic parenchyma to the jejunum. Following an enterotomy, interrupted 5-0 PDS sutures are used to approximate jejunal mucosa to the pancreatic duct. A small stent is placed after the posterior duct-to-mucosa stitches and before the anterior ones. A final anterior layer of 2-0 silk buttress sutures completes the anastomosis. Attention is next turned to the hepaticojejunostomy, which is performed using two 4-0 V-loc sutures in a running fashion. Finally, an antecolic end to side hand-sewn gastrojejunostomy is performed by using 2-0 silk to place interrupted mattress stitches in the outer layer. The inner layer is performed 3-0 V-loc suture in a running Connell fashion. A Jackson-Pratt drains is left anterior to the pancreaticojejunostomy at the conclusion of the procedure. The instruments are removed and the robot is undocked. The abdominal cavity is suctioned and irrigated out. All incisions are closed in layers.
Video clinical vignette
Our video shows a 58-year-old female who presented with painless jaundice and was diagnosed with cancer of the head of the pancreas. At diagnosis, the tumor measured 38×32 mm2 by EUS and abutted the SMA and SMV. Her CA 19-9 level was 56. She was started on neoadjuvant chemotherapy, (completed 4 cycles of FOLFORINOX and 2 cycles of gemcitabine/Abraxane) demonstrating a partial radiographic response with tumor regression to a size of 21×8 mm2 and no longer abutting the SMA. Her post-chemo CA 19-9 level was 15. The patient underwent a robotic pancreaticoduodenectomy with partial venous resection of a 5-cm segment of the lateral wall of the Portal vein/SMV junction and bovine patch reconstruction. Total vascular clamp time was 55 minutes. There were no intra-operative complications. The final pathology showed a 0.8 cm moderately differentiated pancreatic ductal adenocarcinoma invading the peripancreatic tissues, with minimal-to-moderate treatment response, no lymphovascular or perineural invasion, carcinoma involving 2 of 23 nodes and negative margins of resection with a final pathological stage IIB (ypT3 N1 M0). The patient had no post-operative complications, the peripancreatic drain was removed on post-op day 3 and the patient was discharged home on post-op day 7.
Post-operative management
The post-operative management of patients after pancreatic resections with vascular reconstructions is similar to that of our standard robotic pancreaticoduodenectomies. Postoperatively, patients are taken to the surgical floor and the ICU is reserved for patients with significant co-morbidities or per the discretion of the attending surgeon (20). Patients ambulate as early as the evening of the surgery. Nasogastric tubes are not routinely used post-operatively. Patients are kept nothing per os (NPO) the night of surgery, and sips of clear liquids are started on post-operative day (POD) 1. Diets are advanced as tolerated. After vascular reconstructions, patients are given regular strength Aspirin daily starting in the recovery room, initially rectally and subsequently in an oral formulation once they are tolerating a diet. All patients receive chemical deep venous thrombosis (DVT) prophylaxis with subcutaneous heparin prior to surgery, and it is continued post-operatively starting the night of surgery. Patients are closely monitored for any evidence of mesenteric venous hypertension. One of the earliest signs of this is abrupt increase in the volume of the surgical drain with clear non-amylase, non-bilious, non-chylous fluid. A duplex ultrasound can be attained to assess flow of the meso-portal system. If there is any evidence of mesenteric venous hypertension or PV/SMV thrombosis, patients are started on a heparin drip. In our experience, early PV/SMV thrombosis is best treated with systemic anticoagulation and we avoid operative re-exploration as it rarely is able to re-establish a patent graft.
The most common complication affecting patients following pancreatic surgery is a pancreatic fistula. Our standard approach is to leave one drain anterior to the pancreatic anastomosis following completion of surgery. Drain amylase is checked on POD 1 and 3. The drain is removed on POD 3 if: (I) drain amylase is less than 5,000 IU on POD 1 and decreasing by POD 3; (II) the volume of the drain output is less than 500 cc/day; (III) the fluid appearance is non-bilious, non-chylous. The second most common complication is delayed gastric emptying (DGE). DGE results from a functional impairment of gastric motility, resulting in delay to oral intake, prolongation of hospital-stay and poor quality of life. Development of pancreatic leak, post-operative sepsis and need for reoperation are independent risk factors for DGE following pancreaticoduodenectomy (21). Our median length of stay for a patient on the ERAS pathway is 6 days. Patients follow up in the office 2 to 3 weeks after surgery to assess resolution of pancreatic fistulas, discuss final histopathology and to outline a plan for adjuvant therapy as indicated.
Conclusions
The role of minimally invasive pancreatic surgery for pancreas cancer with vascular involvement at the time of surgery is likely to expand as surgeons become more comfortable with Minimally Invasive Surgery (MIS) platforms. Available data stems from high-volume institutional retrospective reviews (22-24), that demonstrate similar operative and oncologic outcomes for MIS compared to standard open pancreatic surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yekebas EF, Bogoevski D, Cataldegirmen G, et al. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and long-term survival in 136 patients. Ann Surg 2008;247:300-9. [Crossref] [PubMed]
- Kaneoka Y, Yamaguchi A, Isogai M. Portal or superior mesenteric vein resection for pancreatic head adenocarcinoma: prognostic value of the length of venous resection. Surgery 2009;145:417-25. [Crossref] [PubMed]
- Toomey P, Hernandez J, Morton C, et al. Resection of portovenous structures to obtain microscopically negative margins during pancreaticoduodenectomy for pancreatic adenocarcinoma is worthwhile. Am Surg 2009;75:804-9; discussion 809-10. [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 846-8. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153-9. [Crossref] [PubMed]
- Zureikat AH, Postlewait LM, Liu Y, et al. A Multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg 2016;264:640-9. [Crossref] [PubMed]
- King JC, Zeh HJ 3rd, Zureikat AH, et al. Safety in numbers: progressive implementation of a robotics program in an academic surgical oncology practice. Surg Innov 2016;23:407-14. [Crossref] [PubMed]
- Ocuin LM, Miller-Ocuin JL, Novak SM, et al. Robotic and open distal pancreatectomy with celiac axis resection for locally advanced pancreatic body tumors: a single institutional assessment of perioperative outcomes and survival. HPB (Oxford) 2016;18:835-42. [Crossref] [PubMed]
- Boggi U, Signori S, De Lio N, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg 2013;100:917-25. [Crossref] [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg 2015;19:189-94; discussion 194. [Crossref] [PubMed]
- Kendrick ML, Sclabas GM. Major venous resection during total laparoscopic pancreaticoduodenectomy. HPB (Oxford) 2011;13:454-8. [Crossref] [PubMed]
- Giulianotti PC, Addeo P, Buchs NC, et al. Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas 2011;40:1264-70. [Crossref] [PubMed]
- Muller SA, Hartel M, Mehrabi A, et al. Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg 2009;13:784-92. [Crossref] [PubMed]
- Boone BA, Steve J, Zenati MS, et al. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol 2014;21:4351-8. [Crossref] [PubMed]
- Zureikat AH, Nguyen KT, Bartlett DL, et al. Robotic-assisted major pancreatic resection and reconstruction. Arch Surg 2011;146:256-61. [Crossref] [PubMed]
- Nguyen KT, Zureikat AH, Chalikonda S, et al. Technical aspects of robotic-assisted pancreaticoduodenectomy (RAPD). J Gastrointest Surg 2011;15:870-5. [Crossref] [PubMed]
- Zureikat AH, Hogg ME, Zeh HJ 3rd. The utility of the robot in pancreatic resections. Adv Surg 2014;48:77-95. [Crossref] [PubMed]
- Allan BJ, Novak SM, Hogg ME, et al. Intraoperative video of robotic pancreatic resection with tumor involvement of the SMV-PV junction. Asvide 2018;5:029. Available online: http://asvidett.amegroups.com/article/view/22378
- Cunningham KE, Zenati MS, Petrie JR, et al. A policy of omitting an intensive care unit stay after robotic pancreaticoduodenectomy is safe and cost-effective. J Surg Res 2016;204:8-14. [Crossref] [PubMed]
- Parmar AD, Sheffield KM, Vargas GM, et al. Factors associated with delayed gastric emptying after pancreaticoduodenectomy. HPB (Oxford) 2013;15:763-72. [Crossref] [PubMed]
- Kauffmann EF, Napoli N, Menonna F, et al. Robotic pancreatoduodenectomy with vascular resection. Langenbecks Arch Surg 2016;401:1111-22. [Crossref] [PubMed]
- Podda M, Thompson J, Kulli CT, et al. Vascular resection in pancreaticoduodenectomy for periampullary cancers. A 10 year retrospective cohort study. Int J Surg 2017;39:37-44. [Crossref] [PubMed]
- Chen S, Chen JZ, Zhan Q, et al. Robot-assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid-term follow-up study. Surg Endosc 2015;29:3698-711. [Crossref] [PubMed]
Cite this article as: Allan BJ, Novak SM, Hogg ME, Zeh HJ. Robotic vascular resections during Whipple procedure. J Vis Surg 2018;4:13.


