Predictors of unexpected nodal upstaging in patients with cT1-3N0 non-small cell lung cancer (NSCLC) submitted to thoracoscopic lobectomy
Introduction
Non-small cell lung cancer (NSCLC) is the first cause of cancer-related death in the world, accounting 1.38 million deaths worldwide in 2008 (1).
The constant improvement in the reliability of pre-operative staging, together with the increasing incidence of peripheral lung tumors and the development of new surgical devices, allowed the thoracoscopic approach to rapidly expand from minor diagnostic procedures to curative surgical management of lung cancer, including major pulmonary resections as lobectomies (2,3). Over the past 2 decades, video-assisted thoracoscopic surgery (VATS) has shown superior perioperative outcomes, reduced complication rates, shorter hospital stays, and improved long-term survival rates compared to thoracotomy. Although some authors described its safety also in locally advanced NSCLC and in patients submitted to induction therapy, to date VATS lobectomy is meant to be the gold standard in the treatment of early stage NSCLC (4-9).
As for all type of tumors, in addition to the diagnosis the clinical staging is fundamental for its important implications on the choice of treatment and on the prognosis. In absence of distant metastasis, a mediastinal nodal involvement is a major determinant of treatment and prognosis in patients affected by NSCLC (10-12).
Standard nodal staging is initially performed non-invasively mainly through computed tomography (CT) and positron-emission tomography (PET).
CT has an important role in the description of the primary tumor, however its reliability in lymph node staging is limited. An important review on 3,438 patients, which analyzed the role of CT in the description of thoracic lymph nodes, found a median sensibility and a median specificity of 57% and 82%, respectively (13). PET, on the contrary, while having a suboptimal spatial resolution, has a much bigger effectiveness concerning mediastinal nodal evaluation. A study on 1,045 patients defined its median sensibility and the median specificity on mediastinal nodal evaluation in 84% and 89%, respectively (13). However, false-positive PET results have been shown in patients with co-existent inflammatory or infectious diseases, while PET/CT scanning may be unable to identify metastatic deposits in normal-sized lymph nodes.
The combination of CT and PET/CT allows the formulation of highly reliable clinical diagnostic hypothesis, defining a clinical staging much closer to the pathologic one, reaching a negative predictive value of up to 98% (14).
Despite the impressive progress in preoperative staging techniques, the finding of postoperative unexpected nodal involvement is still a relevant issue. Aim of this study is to identify possible predictors of unexpected nodal upstaging in patients affected by cT1-3N0 NSCLC submitted to VATS lobectomy.
Methods
Data of 345 patients submitted to thoracoscopic lobectomy at our institution between June 2012 and October 2016 were retrospectively reviewed. The staging was based on the 7th edition of the TNM classification of lung cancer (12). Inclusion criteria for our study were diagnosis of NSCLC and clinical stage cT1-3N0 by means of CT and/or PET/CT. Patients were considered as N0 by means of CT for N size <1 cm and by means of PET/CT for nodal maximum standardized uptake value (SUVmax) <2.5. Patients with a diagnosis of secondary lung tumors, benign diseases and sarcomas were excluded from this study; we also excluded all patients who had undergone neoadjuvant treatments, and the ones who were submitted to invasive clinical staging techniques (both endoscopic or surgical).
Finally, we selected 231 cases who met our study inclusion criteria.
All patients underwent a VATS lobectomy with anterior approach, with a systematic radical mediastinal lymphadenectomy according to ESTS guidelines (15) (Figures 1,2).


At our institution, guidelines for the execution of VATS lobectomy are:
- Vision exclusively through the monitor for all the duration of the surgical intervention;
- Service thoracotomy up to 6 cm in length, without costal spreading (with admission of soft tissue retractors);
- Individual treatment of hilar structures;
- Extraction of the specimen with endo-bag.
For each patient we evaluated the glucose uptake in the primary tumor at PET/CT, and the timing between execution of the PET and surgery. We recorded all histopathological data, with pathological staging according to TNM, comprehensive of the eventual number of involved nodal stations. The study was approved by the Institutional Review Board.
Statistical analysis
Data are reported as absolute numbers or mean or median or percentage. In order to evaluate possible predictor factors, bivariate analysis with χ2 of Fischer’s test were used. For the analysis of independent prognostic factors, linear logistic regression was used. A P value <0.05 has been considered as statistically significant.
Results
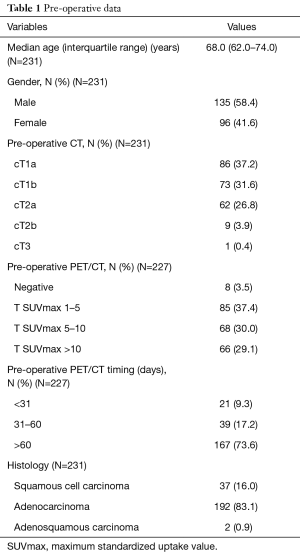
Out of 231 patients, 135 (58.4%) were males and median age was 68.0 (range, 62.0–74.0) years.
Most of the recruited patients were clinically staged as cT1aN0, cT1bN0 (stage IA) and cT2aN0 (stage IB), accounting 86 (37.2%), 73 (31.6%) and 62 (26.8%) subjects, respectively.
Histology was adenocarcinoma in 192 (83.1%) cases, squamous cell carcinoma in 37 (16.0%) patients and adenosquamous carcinoma in 2 (0.9%) cases.
Regarding pre-operative PET assessment, most of our patients had a reported T SUVmax between 1 and 10 (153 patients, 67.4%), 8 (3.5%) patients had a negative PET/CT, whereas a SUVmax over 10 was found in 66 (29.1%) cases. Four patients did not undergo a PET/CT scan. The timing between PET/CT scan assessment and surgery was shorter than 31 days in 21 (9.3%) patients, between 31 and 60 days in 39 (17.2%) cases and over 60 days in 167 (73.6%) subjects (Table 1).
Full table
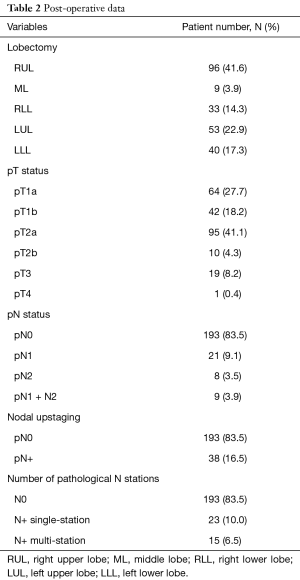
The most frequent operation was the right upper VATS lobectomy (96 patients, 41.6%), followed by left upper lobectomy (53 patients, 22.9%), left lower lobectomy (40 cases, 17.3%), right lower lobectomy (33 patients, 14.3%) and right middle lobectomy (9 cases, 3.9%).
At postoperative staging, the most frequent pathological T (pT) status was pT2a (95 patients, 41.1%).
A nodal upstaging was found in 38 (16.5%) cases. Among these 38 patients, 21 had an exclusively hilar nodal involvement (N1). The number of patients with a pN2 status was 7.4% (17 patients): 8 had merely mediastinal metastasis (N2) and 9 patients had an involvement of both hilar and mediastinal lymph nodes (N1 + N2). Regarding the number of involved nodal stations, 23 patients had single station nodal involvement, whereas 15 had a multiple-station involvement (Table 2).
Full table
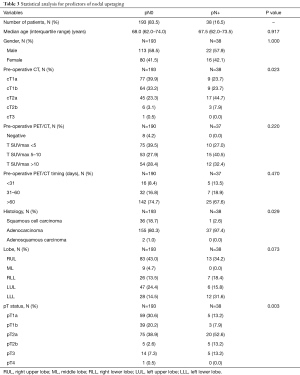
At bivariate analysis for nodal upstaging, the parameters that were statistically significant for prediction of an unexpected nodal upstaging were clinical T (cT) (P=0.023), histology (P=0.029), and pT (P=0.003). Consequently, the likelihood of having nodal upstaging increased at growing of the cT status, and is sensibly bigger for adenocarcinoma compared to squamous cell carcinoma.
A trend towards significance was also observed for left lower lobectomy (Table 3).
Full table
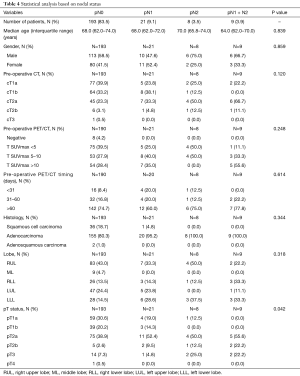
The analysis of factors associated with the type of nodal involvement (i.e., N1, N2 or N1 + N2) showed pT (P=0.042) as the only statistically significant factor (Table 4).
Full table
The analysis based on the number of involved nodal stations (subdivided into pN0, pN+ single-station and pN+ multi-station), found the parameter cT reaching a statistical significance (P=0.030) and so did the parameter pT (P=0.027) (Table 5).
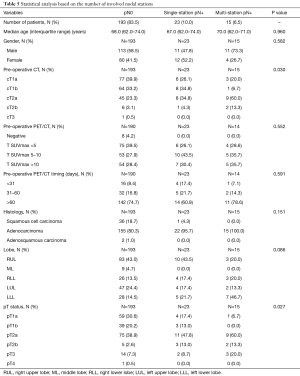
Full table
Finally, at multivariate analysis, adenocarcinoma histology showed to be related to an upstaging risk about 7 times higher than squamous carcinoma. Regarding the pT status, a particularly relevant significance for pT2b was found, which had a predictive value for nodal upstaging almost 11 times higher than pT1a/T1b (Table 6).
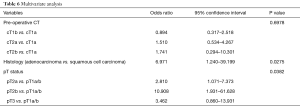
Full table
Discussion
The several advantages of thoracoscopic lobectomy compared to thoracotomy are well known, and in the last decades this approach gained popularity in the treatment of early stage NSCLC also because of the increasing reliability of preoperative staging techniques.
Nevertheless, an under-staging is still a common issue of clinical staging, and particularly N1 or N2 nodal involvement might be an unexpected finding at postoperative histopathological staging.
Many authors analyzed the issue of unexpected nodal upstaging in NSCLC.
On a series of 153 patients clinically staged with CT and PET/CT, Al-Sarraf and colleagues observed 16% occult mediastinal nodal involvement (18). Likewise, Carnochan and Melek reported a 18.4% and 25% occult lymph node involvement rate, respectively (19,20).
In a prospective study aimed to evaluate the real incidence of pN2 in clinical N0 patients, Gomez-Caro and colleagues observed 14.4% of false negative N2 (21). According to this study, the main risk factors for unexpected nodal upstaging were female gender and adenocarcinoma histotype. None of the other considered variables, such as clinical stage, SUV value in the primitive tumor, other patients’ demographic data (i.e., age, comorbidities) showed correlation with the finding of occult nodal metastasis.
As far as a comparison between open versus thoracoscopic surgical approach is concerned, several studies succeeded to demonstrate the reliability of nodal dissection in VATS both in terms of safety and oncological efficacy.
Kim and colleagues showed favorable outcomes when nodal metastasis was incidentally found during thoracoscopic surgery for clinical N0 disease (7.3% false negative N2), with overall and disease-free survival of 89% and 33%, respectively. These rates are comparable to the ones in patients submitted to thoracotomy (22).
In a retrospective study, Wang and colleagues compared early and late outcomes in cN0 patients with postoperative unexpected N2 disease, submitted to VATS lobectomy versus thoracotomy. There was no significant difference neither in the number of dissected lymph nodes, nor in the pathological staging between the two studies’ population. The patients submitted to VATS lobectomy had even better survival rates, which in this case series is explained with a mainly single-station nodal involvement (23).
An analog consideration about single-station nodal disease was highlighted by another study on 1,456 patients, accounting 10.8% of unexpected N+ (24).
In an interesting case series, after undergoing VATS lobectomy with systematical nodal dissection, the patients were submitted to thoracotomy, in order to evaluate the presence of nodal remnants. These were present in only 2–3% of the cases (25).
The equivalent efficacy of VATS lobectomy versus thoracotomy in terms of nodal dissection has been also confirmed by other studies (26,27).
In our case series, the predominance of adenocarcinoma (83.1%) reflects the epidemiological trend, consisting in this histotype’s incidence to be steadily increasing in the last decades, and accounting for about 40% of all lung neoplasms, so replacing squamous cell carcinoma as the most frequent type of NSCLC.
As adenocarcinoma has by definition a prevalent peripheral growth, this intrinsic characteristic makes it particularly favorable for a thoracoscopic approach.
Considering the fact that adenocarcinoma has a greater propensity to give lymphatic metastasis, this can support our result of a more frequent nodal upstaging for this NSCLC subtype.
Our study highlighted a more frequent nodal upstaging in more advanced T stages, thus can be deducted a worsening of prognosis as the tumor dimension raises. This issue has been already addressed and indeed, in the oncoming review of the TNM classification, one of the main items is the subcategorization of the T-parameter (28).
Considering the parallel behavior in the predictive capability between the parameters cT and pT, this can be seen as a sign of a good clinical practice and of a satisfactory reliability of the radiologic staging techniques at our Centre. A good clinical practice can also be deducted by the non-significance of the timing between PEC/CT and surgery in terms of upstaging prediction.
On the other hand, this result might also be explained as the expression of a poorer sensibility of PET/CT in cases of micro-metastatic nodal involvement.
One final consideration can be made regarding the clinical staging. In case of patients with a clinical T2a/T2b/T3 tumor, the application of more invasive staging techniques (endoscopic or/and surgical) could be recommended, as there is an increased chance of nodal upstaging, also in case of a negative radiological staging.
This study has several limitations that must be considered when analyzing its results, particularly the retrospective fashion and the low number of cases analyzed. Moreover, adenocarcinoma was not analyzed in its histological subtypes and similarly the sub-typing of nodal involvement (macro-/micro-metastasis) was not considered.
Conclusions
In patients affected by cT1-3N0 NSCLC submitted to VATS lobectomy, the cT status, the pT status and histology are possible predictors of unexpected nodal upstaging. Adenocarcinoma is associated with a relative risk of unexpected nodal involvement about 7 times higher than squamous cell carcinoma; this relative risk is almost 11 times higher for a pT2b when compared with pT1a/T1b.
Summarizing, for patients affected by cT2a/T2b/T3N0 adenocarcinoma of the lung, we recommend the application of more invasive staging techniques, endoscopic or/and surgical. This recommendation reflects the ESTS guidelines on nodal staging.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
Ethical Statement: The study was approved by the Institutional Review Board of Azienda Ospedaliera di Padova (No. 3812/AO/16). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Charloux A, Quoix E, Wolkove N, et al. The increasing incidence of lung adenocarcinoma: reality or artefact? A review of the epidemiology of lung adenocarcinoma. Int J Epidemiol 1997;26:14-23. [Crossref] [PubMed]
- Heuvelmans MA, Groen HJ, Oudkerk M. Early lung cancer detection by low-dose CT screening: therapeutic implications. Expert Rev Respir Med 2017;11:89-100. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of Thoracoscopic Lobectomy in Locally Advanced Lung Cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Huang J, Xu X, Chen H, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5:S267-73. [PubMed]
- Liu C, Li Z, Bai C, et al. Video-assisted thoracoscopic surgery and thoracotomy during lobectomy for clinical stage I non-small-cell lung cancer have equivalent oncological outcomes: a single-center experience of 212 consecutive resections. Oncol Lett 2015;9:1364-72. [Crossref] [PubMed]
- Nwogu CE, D’Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Cho S, Do YW, Lee EB. Comparison of costs for video-assisted thoracic surgery lobectomy and open lobectomy for non-small cell lung cancer. Surg Endosc 2011;25:1054-61. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical stage I non-small cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Mountain CF. Revisions in the international system for staging lung cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Naruke T, Tsuchiya R, Kondo H, et al. Prognosis and survival after resection for bronchogenic carcinoma based on the 1997 TNM-staging classification: the Japanese experience. Ann Thorac Surg 2001;71:1759-64. [Crossref] [PubMed]
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Toloza EM, Harpole L, McCrory DC. Noninvasive Staging of Non-small Cell Lung Cancer. A Review of the Current Evidence. Chest 2003;123:137S-146S. [Crossref] [PubMed]
- Al-Sarraf N, Gately K, Lucey J, et al. Mediastinal lymph node staging by means of positron emission tomography is less sensitive in elderly patients with non-small-cell lung cancer. Clin Lung Cancer 2008;9:39-43. [Crossref] [PubMed]
- De Leyn P, Doomsb C, Kuzdzalc J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Marulli G, Verderi E, Comacchio GM, et al. Systematic lymph nodes dissection on the right side. Asvide 2018;5:030. Available online: http://asvidett.amegroups.com/article/view/22388
- Marulli G, Verderi E, Comacchio GM, et al. Systematic lymph nodes dissection on the left side. Asvide 2018;5:031. Available online: http://asvidett.amegroups.com/article/view/22389
- Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 2008;33:104-9. [Crossref] [PubMed]
- Carnochan FM, Walker WS. Positron emission tomography may underestimate the extent of thoracic disease in lung cancer patients. Eur J Cardiothorac Surg 2009;35:781-4. [Crossref] [PubMed]
- Melek H, Gunluoglu MZ, Demir A, et al. Role of positron emission tomography in mediastinal lymphatic staging of non-small cell lung cancer. Eur J Cardiothorac Surg 2008;33:294-9. [Crossref] [PubMed]
- Gómez-Caro A, Garcia S, Reguart N, et al. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. Eur J Cardiothorac Surg 2010;37:1168-74. [Crossref] [PubMed]
- Kim HK, Choi YS, Kim J, et al. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non–small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:1288-93. [Crossref] [PubMed]
- Wang S, Zhou W, Zhang H, et al. Feasibility and long-term efficacy of video-assisted thoracic surgery for unexpected pathologic N2 disease in non-small cell lung cancer. Ann Thorac Med 2013;8:170-5. [Crossref] [PubMed]
- Zhong C, Yao F, Zhao H. Clinical Outcomes of Thoracoscopic Lobectomy for Patients With Clinical N0 and Pathologic N2 Non-Small Cell Lung Cancer. Ann Thorac Surg 2013;95:987-92. [Crossref] [PubMed]
- Sagawa M, Sato M, Sakurada A, et al. A prospective trial of systematic nodal dissection for lung cancer by videoassisted thoracic surgery: can it be perfect? Ann Thorac Surg 2002;73:900-4. [Crossref] [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College Wof Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81. [Crossref] [PubMed]
- D’Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
Cite this article as: Marulli G, Verderi E, Comacchio GM, Monaci N, Natale G, Nicotra S, Rea F. Predictors of unexpected nodal upstaging in patients with cT1-3N0 non-small cell lung cancer (NSCLC) submitted to thoracoscopic lobectomy. J Vis Surg 2018;4:15.


