Hybrid treatment of T3 chest wall lung cancer lobectomy
Introduction
The video-assisted thoracoscopic surgery (VATS) has been used since the early 1990s. Over the years, the choice of VATS over thoracotomy in pulmonary resection depends on the personal choice of the surgeon, his experience and the availability of the technical instrument. Non-small cell lung cancer (NSCLC) is now widely treated in VATS, considered in selected cases as a gold standard, though not limited to this type of tumor. In selected patients, 5-year survival without lymph node invasion reaches 40–50% after tumor and chest wall resection, although this procedure may have a higher morbidity with mortality of up to 9%, higher than the simple lobectomy (1-5,6). Unfortunately, in aggressive cases with wall invasion, surgery often involves a potentially disruptive resection, long hospitalization, and a long and painful period of recovery. Several reports have shown that thoracoscopic lobectomy is safe and effective for operable tumors, with overlapping oncological findings and minor complications compared to thoracotomy (5,7-9). Thoracoscopy is not currently the preferred surgery for chest wall invasion because there is not enough studies on the feasibility of lobectomy with wall resection with thoracoscopic hybrid technique.
The purpose of this study was to test a hybrid approach using thoracoscopic lobectomy combined with thoracic wall resection where avoiding rib spreading and scapular mobilization. The best choice in locally advanced disease involving the wall is full lung resection with chest wall to ensure long-term survival. The choice of type of treatment for patients with NSCLC invading the chest wall is under discussion today (10,11-15). The traditional technique used at present is thoracotomy, with wall resection before lobectomy. The parietal resection is done after an exploratory thoracotomy with palpation, which provides a measure of the extent of the disease on the chest wall. This, however, involves an approximation in wall resection. VATS is increasingly gaining ground thanks to many studies in Literature, so the guidelines for its use are being expanded. Our little case shows that using a hybrid approach allows us to engrave the affected area with a precise excision of the chest wall without the need for extensive thoracotomy without rib spreading. Experienced surgeons in VATS demonstrate in Literature the feasibility of the thoracoscopic approach in patients with advanced stage tumor with wall invasion (16).
Patient selection and workup
We selected 3 patients: 2 males and 1 female, smokers, that came to our attention with history of cough and persistent right sided interscapular chest pain (Table 1).
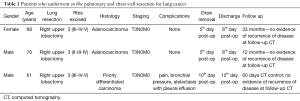
Full table
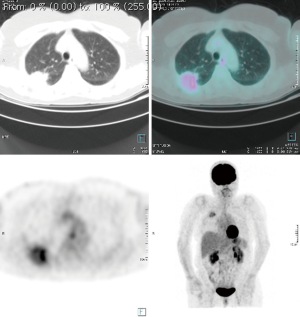
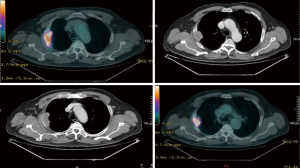
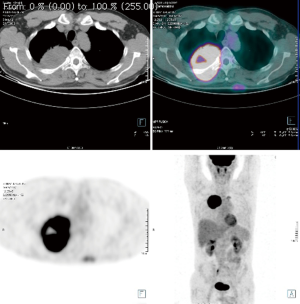
Computed tomography (CT) and positron emission tomography-CT (PET-CT) scans demonstrated a 6.4 cm mass in the right upper lobe invading the segment VI and abutting the pleural surface in the first case, 6 cm same position for the second, 9.4 cm for the third case same position, without hilar adenopathy, CT fine needle biopsy revealing a squamous cell cancer in the first case, a transbronchial biopsy revealed an adenocarcinoma for the second case and a CT fine needle biopsy revealed a poorly differentiated carcinoma in the third case (Figures 1-3).
Pre-operative preparation
Each patient was subjected to induction chemotherapy and two of them concurrent radiotherapy. The induction treatment showed a decrease the T parameter in all patient: to 4.4 cm in the first one, to 2.2 cm in the second and to 6.8 cm in the third (Figures 1-3). All 3 patients were subjected to functional and oncological extended re-evaluation preoperatively after induction treatment [total body contrast CT, PET/CT, brain magnetic resonance imaging (MRI)] and staging standard mediastinoscopy has been performed few days before the major procedure always sampling station 4 L, 7 and 4 R.
Procedure
Patients under general anaesthesia undergo double lumen intubation for one lung ventilation. The patients are harvested with arterial and central versus lines, monitored with capnography, pulse oximetry, ECG and invasive arterial pressure. The patient placed in the lateral decubitus position and homolateral arm in the foreword position but free to be moved in the pending position, three-port anterior VATS approach is performed. This allows for macroscopic staging of the disease and also facilitates location and extension of the chest wall involvement. A right upper lobectomy with complete lobe specific lymph node dissection is performed via conventional anterior 3/5 cm utility VATS incision in the anterior axillary line at the 5th intercostal space. The exact extension of chest wall involvement is assessed under VATS guidance, and the limits of chest wall resection required to achieve adequate clear margins are defined. The patient arm is moved from the foreword position to the pending position to permit the mobilization of the scapula anteriorly and on endoscopic control we can precisely identify the extent of wall resection with the help of peridural needles. Needles are pinned into the chest wall from outside under VATS guidance to mark the extent of chest wall resection. Normally we include at least a 2 cm margin around the tumour for histologic clearance. A needle targeted 10–12 cm posterior incision is performed to allow resection of posterior arches of the ribs, transverse processes and related intercostal musculature without the need of an additional thoracotomy and without rib spreading. The chest wall defect did not need reconstruction in any case. Two intercostal drains are inserted via each VATS port incision and the wound is closed by layers. This approach was used in the three patients (Figure 4). The demographics are described in Table 1.

Role of team members
Each case has been disused in a multidisciplinary meeting dedicated to lung cancer.
Post-operative management
The postoperative outcome is significantly better than the standard surgical procedure. It is mandatory the optimum pain treatment, early mobilization of the patient, respiratory physiotherapy, and last but not least nutrition. In our series the first two patients had no complications at all; the drain removal was in the post-operative 5th day after a chest X-ray. During all the hospitalization all the patients were entrust to the physiotherapist, so early mobilization was achieved. The discharge was without any problem in th 6th day post-surgery. As for the third case, he had pain, bronchial retention (which required fibrotic bronchoscopy), atelectasis with pleural effusion after the surgery, but in the 12th post-operative day, we were able to remove the drainage after the chest X-ray showed a good recovery of the pulmonary panel. We follow the patients with a CT scan after 60 days, and we discharge every patient with a programme as for the pulmonary physiotherapy and for the diagnostic exams (Figures 5,6).
Tips, tricks and pitfalls
We agree that is vital:
- Before approaching the wall resection create a tunnel on the posterior mediastinal pre-esophageal plane and superficially to the Azygos vein, interposing a gauze to protect these structures;
- Intraoperative mobilization of the homolateral arm in a sloping position to anteriorize the scapula to achieve the fullest possible expulsion of the back arches of the affected ribs without the need to raise the bail;
- Use high-energy devices both for dissection and soft wall tissue and to control intercostal arteries;
- Keep the thoracoscope in place for the rest of the procedure to see what happens in the pleural cord;
- At the end of the wall resection, mobilize the homolateral arm to verify that the scapula does not enter the pleural cord;
- In the case of extension to the VI segment, perform the parenchymal section after the wall resection;
- Perform the 4th and 7th linfectomy from the back access at the end of the demolition.
Conclusions
In Literature there are not enough clinical studies that highlight the feasibility of chest wall resection in VATS. The hybrid technique, which includes a VATS lobectomy with chest wall resection, becomes viable and suitable in selected patients.
The mini-invasive technique is certainly applicable to extensive lobectomy to wall resection and can also be reproduced by surgeons with a meaningful experience in the field, as this little series demonstrates. The postoperative outcome is significantly better than the standard surgical procedure. It is mandatory the optimum pain treatment, early mobilization of the patient, respiratory physiotherapy, and last but not least nutrition. The use of more and more specific instrumentation will help reducing the size of the interscope-vertebral access. As this little series, we did not have the need to rebuild the wall defect or to resect the scapula (3,5,18).
There is the need of more studies and experience to accurately determine if using this approach can effectively offer advantages over the classic approach and improve the end result. Selecting the patient with precision is mandatory, considering the size of the tumor, the presence of lymphadenopathy, the possible neo-adjuvant chemotherapy or radiotherapy as in our series, which could entail more technical difficulties by addressing the VATS intervention. Obviously, a mini-invasive approach involves minor alterations of the wall muscles and the ribcage and less nervous trauma than thoracotomy, reducing post-operative pain and improving patient outcome and quality of life (QoL) (1,4,9). In our case, we can’t demonstrate a real advantage of the hybrid approach to the classical one, but only its usefulness and viability, in well-selected patients, which correspond to particular surgical feasibility criteria. Our aim is to propose an approach that could be inspirational for other studies on cancer patients with chest wall invasion and could push to choice this type of treatment only after gaining considerable experience in VATS. The purpose of this type of study should always be to improve the QoL of patients. VATS in patients with lung cancer invading the wall allows for complete resection, less hospitalization and rapid healing. The use of hypodermic needles to delineate the wall resection limits in VATS has allowed us to reschedule the exact amount of chest wall avoiding thoracotomy with optimal neuromuscular and respiratory recovery. This simple approach is repeatable in any case of invasion of the chest wall in selected patients.
In our experience it has been successfully used according to the previous experience described by D’Amico (5). A major diffusion of this technique is necessary in order to better point out its advantages. An optimal learning curve looks inevitable so to bring the surgeon to obtain an adequate experience to complete this type of surgical approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kawaguchi T, Tojo T, Kawai N, et al. A new minimally invasive technique of combined chest wall resection for lung cancer. Surg Today 2016;46:1348-51. [Crossref] [PubMed]
- Kara HV, Balderson SS, D'Amico TA. Challenging cases: thoracoscopic lobectomy with chest wall resection and sleeve lobectomy-Duke experience. J Thorac Dis 2014;6:S637-40. [PubMed]
- Gonfiotti A, Bongiolatti S, Borgianni S, et al. Development of a video-assisted thoracoscopic lobectomy program in a single institution: results before and after completion of the learning curve. J Cardiothorac Surg 2016;11:130. [Crossref] [PubMed]
- Gonfiotti A, Bongiolatti S, Viggiano D, et al. Does videomediastinoscopy with frozen sections improve mediastinal staging during video-assisted thoracic surgery pulmonary resections? J Thorac Dis 2016;8:3496-504. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Lococo F, Rapicetta C, Cardillo G, et al. Pathologic Findings and Long-Term Results After Surgical Treatment for Pulmonary Sarcomatoid Tumors: A Multicenter Analysis. Ann Thorac Surg 2017;103:1142-50. [Crossref] [PubMed]
- Caruana EJ, Solli P, Coonar AS. Hybrid video-assisted thoracoscopic surgery lobectomy and en-bloc chest wall resection for non-small cell lung cancer. J Thorac Dis 2016;8:E935-7. [Crossref] [PubMed]
- Cardillo G, Spaggiari L, Galetta D, et al. Pneumonectomy with en bloc chest wall resection: is it worthwhile? Report on 34 patients from two institutions. Interact Cardiovasc Thorac Surg 2013;17:54-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Single-incision thoracoscopic right upper lobectomy with chest wall resection by posterior approach. Innovations (Phila) 2013;8:70-2. [Crossref] [PubMed]
- Ramella S, Fiore M, Silipigni S, et al. Local Control and Toxicity of Adaptive Radiotherapy Using Weekly CT Imaging: Results from the LARTIA Trial in Stage III NSCLC. J Thorac Oncol 2017;12:1122-30. [Crossref] [PubMed]
- Rosso L, Nosotti M, Palleschi A, et al. VATS lobectomy combined with limited Shaw-Paulson thoracotomy for posterolateral Pancoast tumor. Tumori 2016.102. [PubMed]
- Matsuoka H, Nishio W, Okada M, et al. Resection of chest wall invasion in patients with non-small cell lung cancer. Eur J Cardiothorac Surg 2004;26:1200-4. [Crossref] [PubMed]
- Bayarri CI, de Guevara AC, Martin-Ucar AE. Initial single-port thoracoscopy to reduce surgical trauma during open en bloc chest wall and pulmonary resection for locally invasive cancer. Interact Cardiovasc Thorac Surg 2013;17:32-5. [Crossref] [PubMed]
- Giaccone A, Solli P, Pardolesi A, et al. Video-assisted thoracoscopic surgery en bloc chest wall resection. J Vis Surg 2017;3:73. [Crossref] [PubMed]
- Yendamuri S, Nwogu CE, Demmy TL. Thoracoscopic lobectomy with chest wall resection after neoadjuvant therapy. Innovations (Phila) 2009;4:36-8. [Crossref] [PubMed]
- Widmann MD, Caccavale RJ, Bocage JP, et al. Video-assisted thoracic surgery resection of chest wall en bloc for lung carcinoma. Ann Thorac Surg 2000;70:2138-40. [Crossref] [PubMed]
- Jaus MO, Forcione A, Gonfiotti A, et al. Tecnical surgical procedure for hybrid treatment of T3 chest wall lung cancer lobectomy. Asvide 2018;5:073. Available online: http://asvidett.amegroups.com/article/view/22834
- Lin YT, Hsu PK, Hsu HS, et al. En bloc resection for lung cancer with chest wall invasion. J Chin Med Assoc 2006;69:157-61. [Crossref] [PubMed]
Cite this article as: Jaus MO, Forcione A, Gonfiotti A, Carleo F, De Massimi AR, Carbone L, Di Martino M, Cardillo G. Hybrid treatment of T3 chest wall lung cancer lobectomy. J Vis Surg 2018;4:32.